

铜催化加成/取代串联反应构建四取代的三吲哚甲烷
收稿日期: 2020-07-23
修回日期: 2020-08-21
网络出版日期: 2020-09-22
基金资助
国家自然科学基金(U1504206); 中国博士后科学基金(2020M672200); 河南大学研究生教育创新与质量提升计划(SYL19060137); 河南大学研究生教育创新与质量提升计划(SYL19030204)
Access to Tetrasubstituted Tri(indolyl)methanes through Copper-Catalyzed Addition/Substitution Sequence
Received date: 2020-07-23
Revised date: 2020-08-21
Online published: 2020-09-22
Supported by
the National Natural Science Foundation of China(U1504206); the China Postdoctoral Science Foundation(2020M672200); the Graduate Education Innovation and Quality Improvement Program of Henan University(SYL19060137); the Graduate Education Innovation and Quality Improvement Program of Henan University(SYL19030204)
王文博 , 韩华彬 , 王乐乐 , 王琪琳 , 卜站伟 . 铜催化加成/取代串联反应构建四取代的三吲哚甲烷[J]. 有机化学, 2021 , 41(2) : 757 -765 . DOI: 10.6023/cjoc202007055
An efficient one-pot synthesis of highly crowded tetrasubstituted tri(indolyl)methanes has been achieved through the addition/substitution sequence of 3-acetylindoles and indoles under mild conditions. The key to the success was improving the reactivity of 3-acetylindoles by rationally installing the N-protecting groups. The salient features including easily accessible and low-cost starting materials, board substrate scope, simple operation and structurally useful products make this approach particularly attractive.
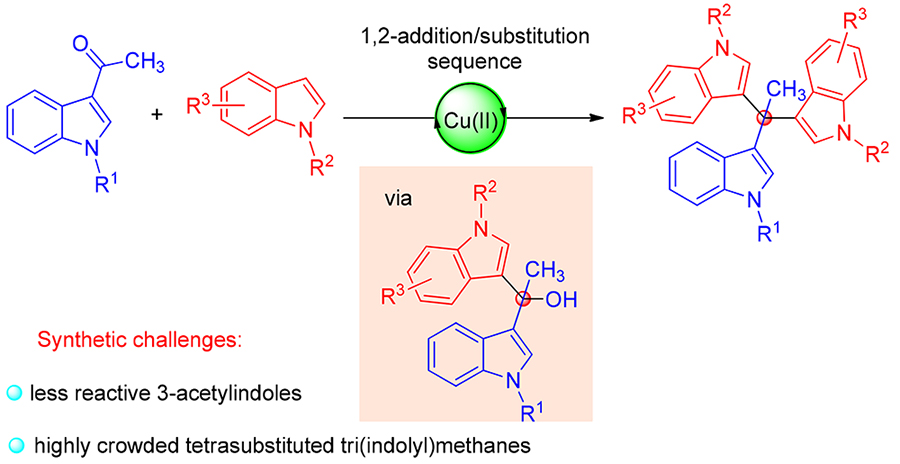
Key words: indole; tri(indolyl)methane; cascade reaction
| [1] | For selected reviews, see: a Somei, M.; Yamada, F.Nat. Prod. Rep. 2005, 22, 73. |
| [1] | (b) Cacchi S.; Fabrizi G. Chem. Rev. 2005, 105, 2873. |
| [1] | (c) Chen F.E.; Huang J. Chem. Rev. 2005, 105, 4671. |
| [1] | (d) Humphrey G.R.; Kuethe J.T. Chem. Rev. 2006, 106, 2875. |
| [1] | (e) Zhang Y.C.; Jiang F.; Shi F. Acc. Chem. Res. 2020, 53, 425. |
| [2] | For selected examples, see: a Jiang, F.; Chen, K. W.; Wu, P.; Zhang, Y. C.; Jiao, Y. C.; Shi, F.Angew. Chem., Int. Ed. 2019, 58, 15104. |
| [2] | (b) Sun M.; Ma C.; Zhou S.J.; Lou S.F.; Xiao J.; Jiao Y.C.; Shi F. Angew. Chem., Int. Ed. 2019, 58, 8703. |
| [2] | (c) Wang H.Q.; Xu M.M.; Wan Y.; Mao Y.J.; Mei G.J.; Shi F. Adv. Synth. Catal. 2018, 360, 1850. |
| [2] | (d) Bai G.X.; Dong F.Y.; Xu L.B.; Liu Y.J.; Wang L.; Li S.S. Org. Lett. 2019, 21, 6225. |
| [2] | (e) Liu X.L.; Zhou G.; Gong Y.; Yao Z.; Zuo X.; Zhang W.H.; Zhou Y. Org. Lett. 2019, 21, 2528. |
| [2] | (f) Xu S.W.; Liu X.W.; Zuo X.; Zhou G.; Gong Y.; Liu X.L.; Zhou Y. Adv. Synth. Catal. 2019, 361, 5328. |
| [2] | (g) Zhu Z.Q.; Yin L.; Wang Y.; Shen Y.; Li C.; Mei G.J.; Shi F. Org. Chem. Front. 2017, 4, 57. |
| [2] | (h) Wang X.; Li G.F.; Sun K.; Zhang B. Chin. J. Org. Chem. 2020, 40, 913. (in Chinese) |
| [2] | 王薪, 李国锋, 孙凯, 张冰, 有机化学, 2020, 40, 913.). |
| [2] | (i) Sun K.; Li Y.L.; Feng R.R.; Mu S.Q.; Wang X.; Zhang B. J. Org. Chem. 2020, 85, 1001. |
| [2] | (j) Wang X.; Wang Q.L.; Xue Y.R.; Sun K.; Wu L.L.; Zhang B. Chem. Commun. 2020, 56, 4436. |
| [2] | (k) Sun K.; Li G.F.; Li Y.Y.; Yu J.; Zhao Q.; Zhang Z.G.; Zhang G.S. Adv. Synth. Catal. 2020, 362, 1947. |
| [3] | (a) Giuseppe B.; Ines B.; Raffaele R.; Jacques L.; Gevenieve B. J. Nat. Prod. 1995, 58, 1254. |
| [3] | (b) Chang Y.C.; Riby J.; Chang G. H. F.; Peng B.C.; Firestone G.; Bjeldanes L.F. Biochem. Pharmacol. 1999, 58, 825. |
| [3] | (c) Lee C.H.; Yao C.F.; Huang S.M.; Ko S.K.; Tan Y.H.; Lee-Chen G.J.; Wang Y.C. Cancer 2008, 113, 815. |
| [3] | (d) Kamal A.; Srikanth Y. V. V.; Khan M. N. A.; Shaik T.B.; Ashraf M. Bioorg. Med. Chem. Lett. 2010, 20, 5229. |
| [4] | (a) Garbe T.R.; Kobayashi M.; Shimizu M.; Takesue N.; Ozawa M.; Yukawa H. J. Nat. Prod. 2000, 63, 596. |
| [4] | (b) Mason M.R.; Barnard T.S.; Segla M.F.; Xie B.H.; Kirschbaum K. J. Chem. Crystallogr. 2003, 33, 531. |
| [5] | (a) Zeng X.F.; Ji S.J.; Su X.M. Chin. J. Chem. 2008, 26, 413. |
| [5] | (b) Chakrabarty M.; Sarkar S.; Linden A.; Stein B.K. Synth. Commun. 2004, 34, 1801. |
| [5] | (c) Zhang Z.H.; Lin J. Synth. Commun. 2007, 37, 209. |
| [5] | (d) Akgün E.; Pindur U.; Müller J. J. Heterocycl. Chem. 1983, 20, 1303. |
| [6] | Bergman J. J. Heterocycl. Chem. 1971, 8, 329. |
| [7] | Seli? L.; Stanovnik B. Tetrahedron 2001, 57, 3159. |
| [8] | (a) El Sayed, M.T.; Ahmed, K.M.; Mahmoud, K.; Hilgeroth, A. Eur. J. Med. Chem. 2015, 90, 845. |
| [8] | (b) Noland W.E.; Kumar H.V.; Flick G.C.; Aapros C.L.; Yoon J.H.; Wilt A.C.; Dehkordi N.; Thao S.; Schneered A.K.; Gao S.M.; Tritch K.J. Tetrahedron 2017, 73, 3913. |
| [8] | (c) Chakrabarty M.; Sarkar S. Tetrahedron Lett. 2002, 43, 1351. |
| [8] | (d) Veisi H.; Maleki B.; Eshbala F.H.; Veisi H.; Masti R.; Ashrafi S.S.; Baghayeri M. RSC Adv. 2014, 4, 30683. |
| [8] | (e) Gu D.G.; Ji S.J. Chin. J. Chem. 2008, 26, 578. |
| [9] | Xiang J.C.; Wang J.G.; Wang M.; Meng X.G.; Wu A.X. Org. Biomol. Chem. 2015, 13, 4240. |
| [10] | (a) El Sayed, M.T.; Mahmoud, K.; Hilgeroth, A.; Fakhr, I. M. I. J. Heterocycl. Chem. 2016, 53, 188. |
| [10] | (b) Khaksar S.; Vahdat S.M.; Gholizadeh M.; Talesh S.M. J. Fluorine Chem. 2012, 136, 8. |
| [11] | Wang W.B.; Zhu Y.S.; Guo S.Q.; Wang Q.L.; Bu Z.W. Org. Biomol. Chem. 2016, 14, 4420. |
| [12] | (a) Zhu Y.S.; Zhou J.; Jin S.J.; Dong H.H.; Guo J.M.; Bai X.G.; Wang Q.L.; Bu Z.W. Chem. Commun. 2017, 53, 11201. |
| [12] | (b) Wang W.B.; Bai X.G.; Jin S.J.; Guo J.M.; Zhao Y.; Miao H.J.; Zhu Y.S.; Wang Q.L.; Bu Z.W. Org. Lett. 2018, 20, 3451. |
| [12] | (c) Guo J.M.; Miao H.J.; Zhao Y.; Bai X.G.; Zhu Y.S.; Wang Q.L.; Bu Z.W. Chem. Commun. 2019, 55, 5207. |
| [12] | (d) Zhang K.; Han H.B.; Wang L.L.; Zhang Z.Y.; Wang Q.L.; Zhang W.J.; Bu Z.W. Chem. Commun. 2019, 55, 13681. |
| [12] | (e) Wang L.L.; Han H.B.; Cui Z.H.; Zhao J.W.; Bu Z.W.; Wang Q.L. Org. Lett. 2020, 22, 873. |
| [12] | (f) Miao H.J.; Wang L.L.; Han H.B.; Zhao Y.D.; Wang Q.L.; Bu Z.W. Chem. Sci. 2020, 11, 1418. |
| [12] | (g) Bai X.G.; Miao H.J.; Zhao Y.; Wang Q.L.; Bu Z.W. Org. Lett. 2020, 22, 5068. |
| [13] | Mo L.P.; Ma Z.C.; Zhang Z.H. Synth. Commun. 2005, 35, 1997. |
/
| 〈 |
|
〉 |