

吡啶联噁唑酰胺类化合物的设计、合成及杀菌活性
收稿日期: 2020-07-03
修回日期: 2020-09-23
网络出版日期: 2020-10-12
基金资助
浙江省绿色农药2011协同创新中心开放基金资助项目.
Design, Synthesis and Fungicidal Activities of Pyridyl Oxazoamide Compounds
Received date: 2020-07-03
Revised date: 2020-09-23
Online published: 2020-10-12
陈澍
,
任朝丽
,
田晓雨
,
张冬林
,
杨森
,
袁绍顷
,
杜晓华
,
谭成侠
. 吡啶联噁唑酰胺类化合物的设计、合成及杀菌活性[J]. 有机化学, 2021
, 41(2)
: 842
-848
.
DOI: 10.6023/cjoc202007009
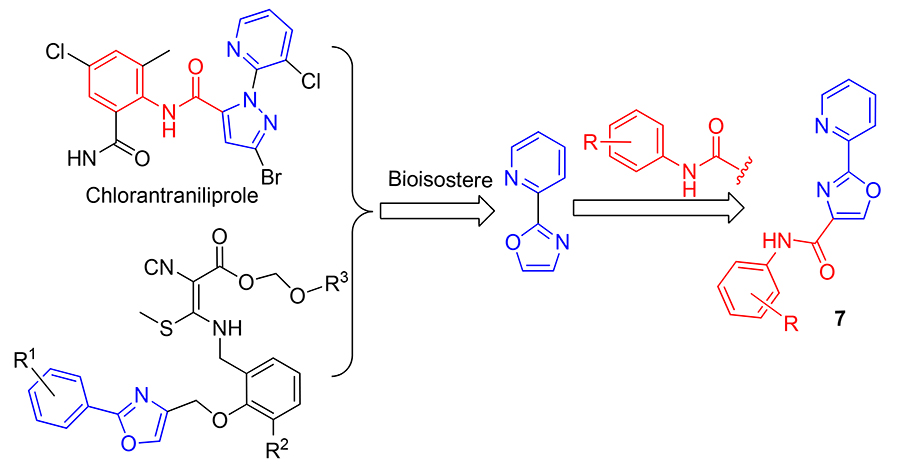
Thirteen novel pyridyl oxazoamide compounds were synthesized using pyridine-2-formaldehyde as the starting material through cyclization, oxidation, substitution, hydrolysis, chlorination and ammonolysis reactions via the disclosed intermediate derivatization method (IDM). The structures of title compounds were characterized by 1H NMR and HRMS. Preliminary bioassay results indicated that at 100 mg/L, 7 compounds exhibited 100% fungicidal activities against Botrytis cinerea, and N-(4-fluorophenyl)-2-(pyridin-2-yl)oxazole-4-carboxamide (7b) exhibited 100% fungicidal activities against Rhizoctonia solani. Further fungicidal activity test results suggested that some of the target compounds had better fungicidal activity than azoxystrobin, which was worthy of further study.
Key words: oxazole; amide; pyridine; fungicide activity
| [1] | Yang J.C.; Guan A.Y.; Yang F.; Liu C.L. Modern Agrochem. 2015, 14, 1. (in Chinese) |
| [1] | 杨吉春, 关爱莹, 杨帆, 刘长令, 现代农药, 2015, 14, 1.). |
| [2] | Wu Z.B.; Kuang J.Q.; Yin J.; Wu S.X.; Cai H. Guizhou Agric. Sci. 2013, 41, 93. (in Chinese) |
| [2] | 吴志兵, 邝继清, 尹娟, 吴世喜, 蔡桦, 贵州农业科学, 2013, 41, 93.). |
| [3] | Kaspady M.; Narayanaswamy V.K.; Raju M.; Rao G.K. Lett. Drug Des. Discovery 2009, 6, 21. |
| [4] | Li Y.; Zhang H.Q.; Liu J.; Yang X.P.; Liu Z.J. J. Agric. Food Chem. 2006, 54, 3636. |
| [5] | Dai H.; Zhuang. H. Y.; Shi, L.; Li, G.; Zhang, H.J.; Fang, Y.; Dai, B.J.Chin. J. Org. Chem. 2015, 35, 2399. (in Chinese) |
| [5] | 戴红, 庄辉阳, 施磊, 李刚, 张海军, 方源, 戴宝江, 有机化学, 2015, 35, 2399.). |
| [6] | Dai H.; Li Y.Q.; Du D.; Qin X.; Zhang X.; Yu H.B.; Fang J.X. J. Agric. Food Chem. 2008, 56, 10805. |
| [7] | Dai H.; Li H.; Jin Z.C.; Liu W.Y.; Xiao Y.; He H.B.; Wang Q.M.; Shi Y.J. Chin. J. Org. Chem. 2016, 36, 185. (in Chinese) |
| [7] | 戴红, 李宏, 金智超, 刘文永, 肖瑶, 何海兵, 汪清民, 石玉军, 有机化学, 2016, 36, 185.). |
| [8] | Yang Y.G.; Meng S.Q.; Qi Z.Q.; Ji M.S.; Li X.H. Chin. J. Pestic. Sci. 2018, 20, 287. (in Chinese) |
| [8] | 杨永贵, 孟司奇, 祁之秋, 纪明山, 李兴海, 农药学学报, 2018, 20, 287.). |
| [9] | Wu J.; Song B.A.; Kang S.D.; Yang S.; Hu D.Y. Pesticides 2012, 51, 625. (in Chinese) |
| [9] | 吴剑, 宋宝安, 康圣鸿, 杨松, 胡德禹, 农药, 2012, 51, 625.). |
| [10] | Liu X.H.; Zhao W.; Shen Z.H.; Xing J.H.; Yuan J.; Yang G.; Xu T.M.; Peng W.L. Bioorg. Med. Chem. Lett. 2016, 26, 3626. |
| [11] | Liu X.H.; Zhao W.; Shen Z.H.; Xing J.H.; Xu T.M.; Peng W.L. Eur. J. Med. Chem. 2017, 125, 881. |
| [12] | Lahm G.P.; Cordova D.; Barry J.D. Bioorg. Med. Chem. 2009, 17, 4127. |
| [13] | Ji W.J.; Xu T.M.; Zheng Z.W.; Zhu B.C.; Li J.; Hu W.Q.; Kong X.L. Chin. J. Pestic. Sci. 2013, 15, 393. (in Chinese) |
| [13] | 姬文娟, 许天明, 郑志文, 朱冰春, 李姣, 胡伟群, 孔小林, 农药学学报, 2013, 15, 393.). |
| [14] | Ery?lmaz S.; ?eliko?lu E.T.; ?dil ?.; ?nkaya E.; Kozak Z.; M?s?r E.; Gül M. Bioorg. Chem. 2020, 95, 103476. |
| [15] | Zhong B.; Liu C.L.; Zhao W.G.; Li Z.M. Chin. J. Org. Chem. 2004, 24, 204. (in Chinese) |
| [15] | 钟滨, 刘长令, 赵卫光, 李正名, 有机化学, 2004, 24, 204.). |
| [16] | Ouyang G.P.; Cai X.J.; Chen Z.; Song B.A.; Bhadury P.S.; Yang S.; Jin L.H.; Xue W.; Hu D.Y.; Zeng S. J. Agric. Food Chem. 2008, 56, 10160. |
| [17] | Wang X.; Wang C.Q.; Fu C.R.; Zou X.M. Chin. J. Org. Chem. 2015, 35, 92. (in Chinese) |
| [17] | 王鑫, 王朝强, 傅翠蓉, 邹小毛, 有机化学, 2015, 35, 92.). |
| [18] | Wang M.M.; Zhang Q.Q.; Yue K.; Li Q.S.; Xu F.B. Chin. J. Org. Chem. 2017, 37, 1774. (in Chinese) |
| [18] | 王梦梦, 张青青, 岳凯, 李庆山, 徐凤波, 有机化学, 2017, 37, 1774.). |
| [19] | Gideens A.C.; Boshoff H. I. M.; Franzblau S.G.; Barry III C.E.; Coppa B.R. Tetrahedron Lett. 2005, 46, 7355. |
| [20] | Prakash T.B.; Reddy G.D.; Padmaja A.; Padmavathi V. Eur. J. Med. Chem. 2014, 82, 347. |
| [21] | Lin J.; Chen J.W.; Cai X.Y.; Qiao X.L.; Huang L.P.; Wang D.G.; Wang Z. J. Agric. Food Chem. 2007, 55, 7626. |
| [22] | Shi Y.J.; Du X.C.; Wang X.L.; Chen Q.W.; Li L.; Dai H.; Xu C.Q.; Zhang J, Y.; Ling, Y.Chin. J. Org. Chem. 2018, 38, 1772. (in Chinese) |
| [22] | 石玉军, 杜显超, 王祥龙, 陈庆文, 李玲, 戴红, 徐蔡芹, 张敬远, 凌勇, 有机化学, 2018, 38, 1772.). |
| [23] | Liu T.T.; Ni Y.; Zhong L.K.; Huang H.Y.; Hu W.Q.; Xu T.M.; Tan C.X. Chin. J. Org. Chem. 2015, 35, 422. (in Chinese) |
| [23] | 刘婷婷, 倪芸, 钟良坤, 黄红英, 胡伟群, 许天明, 谭成侠, 有机化学, 2015, 35, 422.). |
| [24] | Ji J.; Lu E.B.; Wan W.T.; Li M.; Lei H.; Ding Y.G.; Xiong J.L. Chem. Bioeng. 2008, 25, 51. (in Chinese) |
| [24] | 戢峻, 鲁尔贝, 万雯婷, 黎明, 雷昊, 丁一刚, 熊家林, 化学与生物工程, 2008, 25, 51.). |
| [25] | Graham T.H. Org. Lett. 2010, 12, 3614. |
| [26] | Credico B.D.; Reginato G.; Gonsalvi L.; Peruzzini M.; Rossin A. Tetrahedron 2011, 67, 267. |
/
| 〈 |
|
〉 |