

微波促进合成苝单酰亚胺类化合物
收稿日期: 2020-08-09
修回日期: 2020-09-27
网络出版日期: 2020-10-15
基金资助
国家自然科学基金(21572044); 国家自然科学基金(21778013); 河北省科技厅(19241303D)
Microwave-Assisted Synthesis of Perylene Monoimide Derivatives
Received date: 2020-08-09
Revised date: 2020-09-27
Online published: 2020-10-15
Supported by
the National Natural Science Foundation of China(21572044); the National Natural Science Foundation of China(21778013); and the Foundation of the Department of Science and Technology of Hebei Province(19241303D)
王雅楠 , 王冲 , 祁禹鸣 , 李国凯 , 李小六 , 王克让 . 微波促进合成苝单酰亚胺类化合物[J]. 有机化学, 2021 , 41(2) : 702 -707 . DOI: 10.6023/cjoc202008010
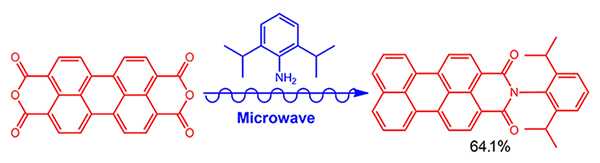
A fast and efficient method for the synthesis of aromatic amine modified perylene monoimide derivatives based on microwave-assisted reaction was developed. It is found that the amount of 2,6-diisopropylaniline showed very important influences on the synthetic yield. N-2',6'-Diisopropylanilino-3,4-perylene monoimide (PMI-1) was obtained with the isolated yield of 64.1% under the optimized condition of 7 equiv. of 2,6-diisopropylaniline at 170 ℃ and 80 W for 30 min.
| [1] | Weil T.; Vosch T.; Hofkens J.; Peneva K.; Müllen K. Angew. Chem. Int. Ed. 2010, 49, 9068. |
| [2] | Bell T. D. M.; Stefan A.; Lemaur V.; Bernhardt S.; Müllen K.; Cornil J.; Beljonne D.; Hofkens J.; der Quweraer M.V.; Schryver F.C. D. Photochem. Photobiol. Sci. 2007, 6, 406. |
| [3] | (a) Sekita M.; Jiménez á.J.; Marcos M.L.; Caballero E.; Rodríguez-Morgade M.S.; Guldi D.M.; Torres T. Chem. - Eur. J. 2015, 21, 19028. |
| [3] | (b) Bolag A.; Sakai N.; Matile S. Chem. - Eur. J. 2016, 22, 9006. |
| [3] | (c) Cheriya R.T.; Joy J.; Alex A.P.; Shaji A.; Hariharan M. J. Phys. Chem. C 2012, 116, 12489. |
| [3] | (d) Li C.; Yan H.; Zhang G.F.; Gong W.L.; Chen T.; Hu R.; Aldred M.P.; Zhu M.Q. Chem. - Asian J. 2014, 9, 104. |
| [4] | Lewandowska U.; Zajaczkowski W.; Pisula W.; Ma Y.; Li C.; Müllen K.; Wennemers H. Chem. - Eur. J. 2016, 22, 3804. |
| [5] | Warnan J.; Willkomm J.; Farré Y.; Pellegrin Y.; Boujtita M.; Odobel F.; Reisner E. Chem. Sci. 2019, 10, 2758. |
| [6] | Kaloyanova S.; Zagranyarski Y.; Ritz S.; Hanulov? M.; Koynov K.; Vonderheit A.; Müllen K.; Peneva K. J. Am. Chem. Soc. 2016, 138, 2881. |
| [7] | Quante H.; Müllen K. Angew. Chem. Int. Ed. Engl. 1995, 34, 1323. |
| [8] | (a) Hutchison J.A.; Uji-I H.; Deres A.; Vosch T.; Rocha S.; Müller S.; Bastian A.A.; Enderlein J.; Nourouzi H.; Li C.; Herrmann A.; Müllen K.; Schryver F.D.; Hofkens J. Nat. Nanotechnol. 2014, 9, 131. |
| [8] | (b) Chen L.; Li C.; Müllen K. J. Mater. Chem. C 2014, 2, 1938. |
| [8] | (c) Schmaltz B.; Weil T.; Müllen K. Adv. Mater. 2009, 21, 1067. |
| [8] | (d) Berberich M.; Würthner F. Chem. Sci. 2012, 3, 2771. |
| [8] | (e) Chen L.; Li C.; Müllen K. J. Mater. Chem. C 2014, 2, 1938. |
| [8] | (f) Schmaltz B.; Weil T.; Müllen K. Adv. Mater. 2009, 21, 1067. |
| [8] | (g) Berberich M.; Würthner F. Chem. Sci. 2012, 3, 2771. |
| [9] | Feiler L.; Langhals H.; Polborn K. Liebigs Ann. 1995, 1229. |
| [10] | (a) Cheriya R.T.; Joy J.; Alex A.P.; Shaji A.; Hariharan; M. J. Phys. Chem. C 2012, 116, 12489. |
| [10] | (b) Li C.; Yan H.; Zhang G.F.; Gong W.L.; Chen T.; Hu R.; Aldred M.P.; Zhu M.Q. Chem. - Asian J. 2014, 9, 104. |
| [10] | (c) Wei H.; Jiang N.; Zhao N.; Zhang Y.; Gao B. Chin. J. Chem. 2014, 32, 356. |
| [11] | Langhals H.; von Unold P.; Speckbacher M. Liebigs Ann./Recl. 1997, 467. |
| [12] | Geerts Y.; Quante H.; Platz H.; Mahrt R.; Hopmeier M.; B?hm A.; Müllen K. J. Mater. Chem. 1998, 8, 2357. |
| [13] | (a) Tr?ster H. Dyes Pigm. 1983, 4, 171. |
| [13] | (b) Tam-Chang S.W.; Seo W.; Iverson I.K. J. Org. Chem. 2004, 69, 2719. |
| [13] | (c) Huang L.; Catalano V.J.; Tam-Chang S.W. Chem. Commun. 2007, 2016. |
| [14] | Chen L.; Zhang K.; Zhu L.; Xiao Y. Ind. Eng. Chem. Res. 2015, 54, 12699. |
| [15] | (a) Yi X.; Zhang Z.; Huang H. Baell J.B.; Yu Y.; Huang F. Chin. J. Org. Chem. 2019, 39, 544. (in Chinese) |
| [15] | 易享炎, 张志鹏, 黄和, Jonathan B. Baell, 于杨, 黄菲, 有机化学, 2019, 39, 544.). |
| [15] | (b) Yan X.; Li J.; Zhang Q.; Shi D. Chin. J. Org. Chem. 2017, 37, 1450. (in Chinese) |
| [15] | 闫小惠, 李加荣, 张奇, 史大昕, 有机化学, 2017, 37, 1450.). |
| [15] | (c) Roberts B.A.; Strauss C.R. Acc. Chem. Res. 2005, 38, 653. |
| [16] | Rigodanza F.; Tenori E.; Bonasera A.; Syrgiannis Z.; Prato M. Eur. J. Org. Chem. 2015, 5060. |
| [17] | Pengo P.; Pantos G.D.; Otto S.; Sanders J. K. M. J. Org. Chem. 2006, 71, 7063. |
| [18] | Tambara K.; Ponnuswamy N.; Hennrich G.; Panto? G.D. J. Org. Chem. 2011, 76, 3338. |
| [19] | Sekita M.; Jiménez á.J.; Marcos M.L.; Caballero E.; Rodríguez-Morgade M.S.; Guldi D.M.; Torres T. Chem. - Eur. J. 2015, 21, 19028. |
| [20] | Chen Z.; Stepanenko V.; Dehm V.; Prins P.; Siebbeles L. D. A.; Seibt J.; Marquetand P.; Engel V.; Würthner F. Chem. - Eur. J. 2007, 13, 436. |
/
| 〈 |
|
〉 |