

四氯化锆催化合成嘧啶并[4,5-b]喹啉-2,4(1H,3H)-二酮和11H-茚[1,2-b]喹啉-11-酮
收稿日期: 2020-07-24
修回日期: 2020-09-30
网络出版日期: 2020-10-22
基金资助
湖北省教育厅重点项目(D20192503); 国家自然科学基金(21542009)
ZrCl4-Catalyzed Synthesis of Pyrimido[4,5-b]quinolin-2,4-(1H,3H)- diones and 11H-Indeno[1,2-b]quinolin-11-ones
Received date: 2020-07-24
Revised date: 2020-09-30
Online published: 2020-10-22
Supported by
Educational Commission of Hubei Province(D20192503); National Natural Science Foundation of China(21542009)
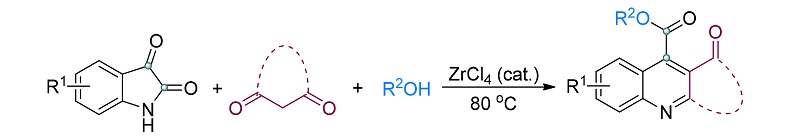
以四氯化锆为催化剂, 靛红、1,3-二甲基巴比妥酸/1,3-茚二酮在脂肪醇中能够通过多组分串联反应, 有效地转化成为相应的稠环化合物嘧啶并[4,5-b]喹啉-2,4(1H,3H)-二酮和11H-茚[1,2-b]喹啉-11-酮, 并巧妙地在这些含氮杂环中引入酯基. 这些未见文献报道的化合物均通过了1H NMR、13C NMR、IR和HRMS进行了表征, X射线单晶衍射确定了11H-茚并[1,2-b]喹啉-11-酮-10-羧酸乙酯(4q)的结构. 这种以简单易得化合物为原料在温和条件下制备上述两类稠环骨架的方法, 是对这些稠环骨架已有合成方法的补充, 可以有效丰富此类杂环化合物库.
殷国栋 , 李源 , 范玲 . 四氯化锆催化合成嘧啶并[4,5-b]喹啉-2,4(1H,3H)-二酮和11H-茚[1,2-b]喹啉-11-酮[J]. 有机化学, 2021 , 41(3) : 1234 -1240 . DOI: 10.6023/cjoc202007056
Using zirconium tetrachloride as the catalyst, a multicomponent tandem reaction of isatin, 1,3-dimethyl barbituric acid/1,3-indanedione and aliphatic alcohol, effectively gave the corresponding fused-ring compounds pyrimido[4,5-b]quino- lone-2,4-(1H,3H)-diones and 11H-indeno[1,2-b]quinolin-11-ones. The ester group was introduced into these nitrogen-containing molecules. All these newly synthesized compounds were identified by means of 1H NMR, 13C NMR, IR and HRMS. Moreover, ethyl-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimido[4,5-b]quinoline-5-carboxylate (4q) was confirmed by X-ray single crystal diffraction analysis. Owing to the mild reaction conditions and simple easy available starting materials, the method for the preparation of the above two fused-ring skeletons is complementary to the existing methods, which can effectively enrich the corresponding heterocyclic compound library.

| [1] | Ismail, M. A.; Al-Shihry, S.; Arafa, R. K.; El-Ayaan, U. J. Enzyme Inhib. Med. Chem. 2013, 28, 530. |
| [2] | Dickens, M. P.; Roxburgh, P.; Hock, A.; Mezna, M.; Kellam, B.; Vousden, K. H.; Fischer, P. M. Bioorg. Med. Chem. 2013, 21, 6868. |
| [3] | Prukala, D.; Gierszewski, M.; Karolczak, J.; Sikorski, M. Phys. Chem. Chem. Phys. 2015, 17, 18729. |
| [4] | (a) Dudkin, S.; Iaroshenko, V. O.; Sosnovskikh, V. Y.; Tolmachev, A. A.; Villinger, A.; Langer, P. Org. Biomol. Chem. 2013, 11, 5351. |
| [4] | (b) Zhang, X. Y.; Guo, X. J.; Fan, X. S. Chem.-Asian J. 2015, 10, 106. |
| [5] | (a) Shen, Q.; Wang, L. M.; Yu, J. J.; Liu, M. T.; Qiu, J.; Fang, L.; Guo, F. L.; Tang, J. Synthesis 2012, 44, 389. |
| [5] | (b) Yang, D. J.; Lv, F.; Guo, W. Chin. J. Org. Chem. 2004, 24, 366. (in Chinese) |
| [5] | (杨定乔, 吕芬, 郭维, 有机化学, 2004, 24, 366.) |
| [6] | (a) Nadaraj, V.; Selvi, S. T.; Mohan, S.; Thangadurai, T. D. Med. Chem. Res. 2012, 21, 2911. |
| [6] | (b) Chandra, A.; Upadhyay, S.; Singh, B.; Sharma, N.; Singh, R. M. Tetrahedron 2011, 67, 9219. 8186ac97-d480-41cb-88a5-97f30b2aff6d |
| [7] | (a) Panday, A. K.; Mishra, R.; Jana, A.; Parvin, T.; Choudhury, L. H. J. Org. Chem. 2018, 83, 3624. |
| [7] | (b) Saikia, P.; Sharma, G.; Gogoi, S.; Boruah, R. C. RSC Adv. 2015, 5, 23210. |
| [8] | (a) Singh, G. S.; Desta, Z. Y. Chem. Rev. 2012, 112, 6104. |
| [8] | (b) Ahmad, M. S.; Zhu, Y. M.; Guo, Y. L.; Zhang, S. S.; Shen, Z. M. Chin. J. Org. Chem. 2019, 39, 3244. (in Chinese) |
| [8] | Ahmad, M. S., 主亚敏, 郭云龙, 张赛赛, 沈增明 2019, 39, 3244.) |
| [9] | (a) Fan, L.; Liu, M. L.; Ye, Y.; Yin, G. D. Org. Lett. 2017, 19, 186. |
| [9] | (b) Liu, M. L.; Shu, M. M.; Yao, C. C.; Yin, G. D.; Wang, D. J.; Huang, J. K. Org. Lett. 2016, 18, 824. |
| [9] | (c) Liu, M.; Qiu, S. Z.; Ye, Y.; Yin, G. D. Tetrahedron Lett. 2016, 57, 5856. |
| [10] | Chakrabarty, S.; Croft, M. S.; Marko, M. G.; Moyna, G. Bioorg. Med. Chem. 2013, 21, 1143. |
| [11] | (a) Peng, X.; Zhu, L.; Hou, Y.; Pang, Y.; Li, Y.; Fu, J.; Yang, L.; Lin, B.; Liu, Y.; Cheng, M. Org. Lett. 2017, 23, 3402. |
| [11] | (b) Rajawinslin, R. R.; Gawande, S. D.; Kavala, V.; Huang, Y. H.; Kuo, C. W.; Chen, M. L.; He, C. H.; Yao, C. F. RSC Adv. 2014, 4, 37806. |
| [12] | Tufail, F.; Saquib, M.; Singh, S.; Tiwari, J.; Singh, M.; Singh, J.; Singh, J. New J. Chem. 2017, 41, 1618. |
| [13] | (a) Zhou, P.; Hu, B.; Wang, Y.; Zhang, Q.; Li, X.; Yan, S.; Yu, F. Eur. J. Org. Chem. 2018, 2018, 4527. |
| [13] | (b) Wang, X.; Yang, Z.; Miu, W.; Ye, P.; Bai, M.; Duan, S.; Shen, X. RSC Adv. 2019, 9, 37057. |
| [14] | (a) Yu, F.; Yan, S.; Hu, L.; Wang, Y.; Lin, J. Org. Lett. 2011, 13, 4782. |
| [14] | (b) Wang, B. Q.; Zhang, C. H.; Tian, X. X.; Lin, J.; Yan, S. J. Org. Lett. 2018, 20, 660. |
| [14] | (c) Shirini, F.; Akbari-Dadamahaleh, S.; Mohammad-Khah, A. Chin. J. Catal. 2013, 34, 2200. |
| [15] | (a) Ton, N. N. H.; Dang, H. V.; Phan, N. T. S.; Nguyen, T. T. RSC Adv. 2019, 9, 16215. |
| [15] | (b) Zhou, P.; Hu, B.; Zhao, S.; Zhao, S.; Zhang, Q.; Wang, Y.; Li, X.; Yu, F. Tetrahedron Lett. 2018, 59, 3116. |
| [15] | (c) Yang, L.; Wan, J. P. Green Chem. 2020, 22, 3074. |
/
| 〈 |
|
〉 |