

以氰甲基亚磷酸酯为含膦试剂的铜催化膦酰化异喹啉酮类化合物的高效合成
收稿日期: 2020-08-25
修回日期: 2020-10-18
网络出版日期: 2020-10-22
基金资助
山东省自然科学基金(ZR2016JL012); 青岛科技大学高科技人才资助项目.
Efficient Copper-Catalyzed Domino Synthesis of Phosphonated Isoquinolin-1(2H)-ones Using Cyanomethylphosphonates as Building Blocks
Received date: 2020-08-25
Revised date: 2020-10-18
Online published: 2020-10-22
Supported by
the Natural Science Foundation of Shandong Province(ZR2016JL012); the Scientific Research Foundation of Qingdao University of Science and Technology.()
赵苏艳 , 宫雪芹 , 甘子玉 , 颜秋莉 , 刘学良 , 杨道山 . 以氰甲基亚磷酸酯为含膦试剂的铜催化膦酰化异喹啉酮类化合物的高效合成[J]. 有机化学, 2021 , 41(1) : 258 -266 . DOI: 10.6023/cjoc202008045
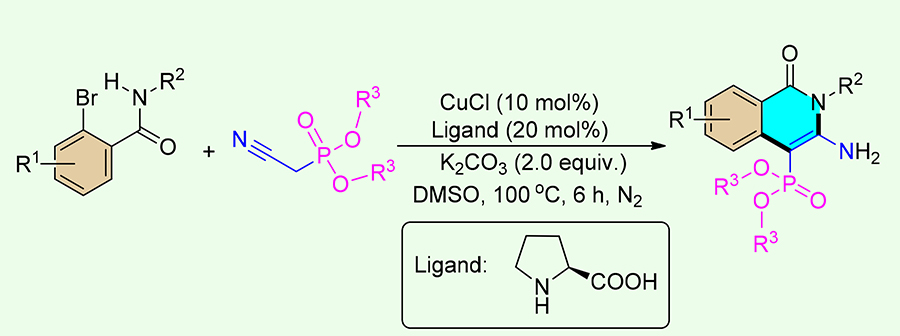
An efficient and convenient copper-catalyzed cascade synthesis of C-4 phosphonated isoquinolin-1(2 H)-ones has been initially proposed. This is the first example for the construction of phosphine-containing heterocycles through copper-catalyzed Ullmann-type coupling reactions using cyanomethylphosphonates as the building blocks, and it will broaden the strategies of organophosphorus synthesis in the field of organic and pharmaceutical chemistry.
| [1] | (a) Ratnayake R.; Lacey E.; Tennant S.; Gill J.H.; Capon R.J. Chem. -Eur. J. 2007, 13, 1610. |
| [1] | (b) Billamboz M.; Bailly F.; Lion C.; Touati N.; Vezin H.; Calmels C.; Andreóla M.-L.; Christ F.; Debyser Z.; Cotelle P. J. Med. Chem. 2011, 54, 1812. |
| [1] | (c) Dickinson R.P.; Bell A.W.; Hitchcock C.A.; Narayana-Swami S.; Ray S.J.; Richardson K.; Troke P.F. Bioorg. Med. Chem. Lett. 1996, 6, 2031. |
| [1] | (d) Jatav V.; Mishra P.; Kashaw S.; Stables J.P. Eur. J. Med. Chem. 2008, 43, 135. |
| [1] | (e) Glushkov V.A.; Shklyaev Y.V. Chem. Heterocycl. Compd. 2001, 37, 66. |
| [2] | (a) Kashaw S.K.; Gupta V.; Kashaw V.; Mishra P.; Stables J.P.; Jain N.K. Med. Chem. Res. 2010, 19, 250. |
| [2] | (b) Wang Z.; He W.-M. Chin. J. Org. Chem. 2019, 39, 3594. (in Chinese) |
| [2] | ( 王峥, 何卫民, 有机化学, 2019, 39, 3594.). |
| [2] | (c) Li X.-L.; Wang J.-Q.; Li L.; Yin Y.-W.; Ye L.-W. Acta Chim. Sinica 2016, 74, 49. (in Chinese) |
| [2] | ( 李新玲, 王佳琪, 李龙, 尹应武, 叶龙武, 化学学报, 2016, 74, 49.). |
| [3] | (a) Horsman G.P.; Zechel D.L. Chem. Rev. 2017, 117, 5704. |
| [3] | (b) Gahungu M.; Arguelles-Arias A.; Fickers P.; Zervosen A.; Joris B.; Damblon C.; Luxen A. Bioorg. Med. Chem. 2013, 21, 4958. |
| [3] | (c) Tang W.; Zhang X. Chem. Rev. 2003, 103, 3029. |
| [3] | (d) Bhattacharya A.K.; Thyagarajan G. Chem. Rev. 1981, 81, 415. |
| [3] | (e) Kirumakki S.; Huang J.; Subbiah A.; Yao J.; Rowland A.; Smith B.; Mukherjee A.; Samarajeewa S.; Clearfield A. J. Mater. Chem. 2009, 19, 2593. |
| [3] | (f) Krylov S.; Kashemirov B.A.; Hilfinger J.M.; McKenna C.E. Mol. Pharmaceutics 2013, 10, 445. |
| [3] | (g) Qiao B.; Cao H.-Q.; Huang Y.-J.; Zhang Y.; Nie J.; Zhang F.-G.; Ma J.-A. Chin. J. Chem. 2018, 36, 809. |
| [4] | (a) Schlummer B.; Scholz U. Adv. Synth. Catal. 2004, 346, 1599. |
| [4] | (b) Hayashi T. Acc. Chem. Res. 2000, 33, 354. |
| [5] | (a) Zhuang R.; Xu J.; Cai Z.; Tang G.; Fang M.; Zhao Y. Org. Lett. 2011, 13, 2110. |
| [5] | (b) Lee H.; Yun J. Org. Lett. 2018, 20, 7961. |
| [5] | (c) Mehellou Y.; Rattan H.S.; Balzarini J. J. Med. Chem. 2018, 61, 2211. |
| [6] | Demmer C.S.; Krogsgaard-Larsen N.; Bunch L. Chem. Rev. 2011, 111, 7981. |
| [7] | Li B.; Yang J.; Xu H.; Song H.; Wang B. J. Org. Chem. 2015, 80, 12397. |
| [8] | (a) Shi W.; Li J.; Su D.; Wang X.; Zhang L.; Pan L.; Wu X.; Wu H. Angew. Chem., Int. Ed. 2019, 58, 1106. |
| [8] | (b) White J.D.; Laura Q.; Wang G. J. Org. Chem. 2007, 72, 1717. |
| [9] | Zhu W.; Zhang D.; Yang N.; Liu H. Chem. Commun. 2014, 50, 10634. |
| [10] | (a) Yang D.; An B.; Wei W.; Tian L.; Huang B.; Wang H. ACS Comb. Sci. 2015, 17, 113. |
| [10] | (b) Yan K.; Yang D.; Wei W.; Lu S.; Li G.; Zhao C.; Zhang Q.; Wang H. Org. Chem. Front. 2016, 3, 66. |
| [10] | (c) Gan Z.; Yan Q.; Li G.; Li Q.; Dou X.; Li G.-Y.; Yang D. Adv. Synth. Catal. 2019, 361, 4558. |
| [11] | (a) Bhunia S.; Pawar G.G.; Kumar S.V.; Jiang Y.; Ma D. Angew. Chem., Int. Ed. 2017, 56, 16136. |
| [11] | (b) Xu S.; Lu J.; Fu H. Chem. Commun. 2011, 47, 5596. |
| [11] | (c) Liu X.; Fu H.; Jiang Y.; Zhao Y. Angew. Chem., Int. Ed. 2009, 48, 348. |
| [11] | (d) Xu L.; Jiang Y.; Ma D. Org. Lett. 2012, 14, 1150. |
| [11] | (e) Ma D.; Cai Q. Acc. Chem. Res. 2008, 41, 1450. |
| [11] | (f) Lv X.; Bao W.L. J. Org. Chem. 2009, 74, 5618. |
| [11] | (g) Martìn R.; Rivero R.; Buchwald S.L. Angew. Chem., Int. Ed. 2006, 45, 7079. |
| [11] | (h) Liu Y.; Wan J.-P. Org. Biomol. Chem. 2011, 9, 6873. |
| [11] | (i) Verma A.K.; Kesharwani T.; Singh J.; Tandon V.; Larock R.C. Angew. Chem., Int. Ed. 2009, 48, 1138. |
| [11] | (j) Moessner C.; Bolm C. Org. Lett. 2005, 7, 2667. |
| [11] | (k) Cheng L.; Ge X.; Liu X.; Feng Y. Chin. J. Org. Chem. 2020, 40, 2008. (in Chinese) |
| [11] | ( 成琳, 葛新, 刘学民, 冯云辉, 有机化学, 2020, 40, 2008.). |
| [11] | (l) Xie J.; Wang X.; Wu F.; Zhang J. Chin. J. Org. Chem. 2019, 39, 3026. (in Chinese) |
| [11] | ( 谢建伟, 汪小创, 吴丰田, 张洁, 有机化学, 2019, 39, 3026.). |
| [12] | (a) Lu B.; Ma D. Org. Lett. 2006, 8, 6115. |
| [12] | (b) Xie X.; Cai G.; Ma D. Org. Lett. 2005, 7, 4693. |
| [12] | (c) Liu T.; Wang R.; Yang H.; Fu H. Chem. -Eur. J. 2011, 17, 6765. |
| [12] | (d) Li M.; Ning J.; Yu L.; Wen L. Chin. J. Org. Chem. 2016, 36, 2715. (in Chinese) |
| [12] | ( 李明, 宁加彬, 于乐, 文丽荣, 有机化学, 2016, 36, 2715.). |
| [12] | (e) Zhao S.; Wang Z.-L. Chin. J. Org. Chem. 2016, 36, 862. (in Chinese) |
| [12] | ( 赵苏艳, 王祖利, 有机化学, 2016, 36, 862.). |
/
| 〈 |
|
〉 |