

烷基铝试剂与亲电试剂的偶联反应研究进展
收稿日期: 2020-09-12
修回日期: 2020-10-13
网络出版日期: 2020-11-04
基金资助
西南民族大学中央高校基本科研业务费(2018NZD06); 四川省科技厅科技支撑计划(2015NZ0033)
Research Progress of Cross-Coupling Reactions of Alkylaluminums with Electrophiles Reagents
Received date: 2020-09-12
Revised date: 2020-10-13
Online published: 2020-11-04
Supported by
Fundamental Research Funds for the Central Universities, the Southwest Minzu University(2018NZD06); Sichuan Provincial Department of Science and Technology Support Program(2015NZ0033)
李清寒 , 罗瑞强 , 吴川 , 肖红柳 , 郭少鹏 , 张志豪 , 黄哲耀 , 周林 . 烷基铝试剂与亲电试剂的偶联反应研究进展[J]. 有机化学, 2021 , 41(4) : 1489 -1497 . DOI: 10.6023/cjoc202009029
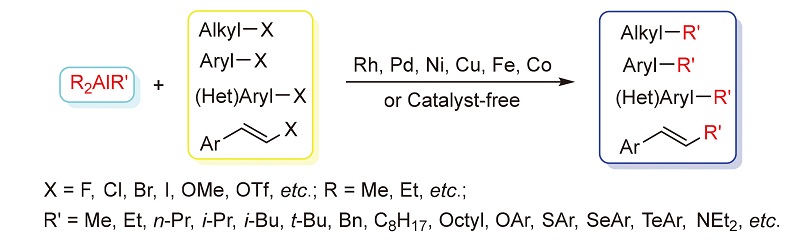
Alkyl aluminum compounds are widely applied in organic reactions because of their high reactivities, low toxicities, and ease of preparation. The cross-coupling reaction of an organoalane compounds with organic electrophiles using a transition-metal catalyst or catalyst-free provides a simple method to synthesize a large variety of compounds and shows a higher functional group tolerance than organolithium and magnesium, which allows for additions to aldehydes in the presence of nitro, ester, hydroxyl, amino, nitrile and lactone moieties. Therefore, many organoalane reagents have found the most applications in cross-coupling reactions in recent years. In this paper, the recent research results about the alkylaluminum reagents applied in cross-coupling reactions are reviewed, involving various reaction systems.
| [1] | Negishi, E-i.; Zeng, X.; Tan, Z.; Qian, M.; Hu, Q.; Huang, Z . Palladium- or Nickel-Catalyzed Cross-Coupling with Organometals Containing Zinc, Aluminum, and Zirconium: The Negishi Coupling. In Metal-Catalyzed Cross-Coupling Reactions, Vol 2, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2004. |
| [2] | Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95,2457. |
| [3] | Kang, S.K.; Yamaguchi, T.; Kim, T.H.; Ho, P.S. J. Org. Chem. 1996, 61,9082. |
| [4] | Cherney, A.H.; Kadunce, N.T.; Reisman, S.E. Chem. Rev. 2015, 115,9587. |
| [5] | Herath, A.; Molteni, V.; Pan, S.F.; Loren, J. Org. Lett. 2018, 20,7429. |
| [6] | Zhu, F.; Wang, Z.X. J. Org. Chem. 2014, 79,4285. |
| [7] | Ranjan, Jana, R.; Pathak, T.P.; Sigman, M.S. Chem. Rev. 2011, 111,1417. |
| [8] | (a) For the synthesis of organoaluminum compounds, see: Knochel, P. Bl?mke, T.; Groll, K.; Chen, Y.-H. Modern Organoaluminum Reagents: Preparation, In Topics in Organometallic Chemistry, Vol. 41, Eds.: Woodward, S.; Dagorne, S., Springer, Berlin, 2013,pp.173~186. |
| [8] | (b) For cross-coupling reactions of organoaluminum compounds, see: Kolb, A. Zezschwitz, P. Modern Organoaluminum Reagents: Preparation, In Topics in Organometallic Chemistry, Vol. 41, Eds.: Woodward, S.; Dagorne, S., Springer, Berlin, 2013, pp.267~276. |
| [9] | Maruoka, K.; Yamamoto, H. Tetrahedron 1988, 44,5001. |
| [10] | Varenikov, A.; Mark Gandelman, M. J. Am. Chem. Soc. 2019, 141,10994. |
| [11] | Li, Q.H.; Shao, X.B.; Ding, Y.; Wen, C.; Zhao, Z.G. Curr. Org. Chem. 2018, 22,1523. |
| [12] | Li, Q.H.; Wang, J.H.; Wen, C.; Xin, J.; Cao, K.P.; Wu, K.; Liang, M. Chin. Chem. Lett. 2019,1830. |
| [13] | Li, Q.H.; Jiang, X.; Wu, K.; Luo, R.Q.; Zhang, Z.H.; Liang, M.; Huang, Z.Y. Mini-Rev. Org. Chem. 2020,. |
| [14] | Li, Q.H.; Shao, X.B.; Zhang, G.; Ding, Y.; Yang, X.J.; Chen, F. Chin. J. Org. Chem. 2018, 38,802. (in Chinese) |
| [14] | ( 李清寒, 杓学蓓, 张刚, 丁勇, 杨学军, 陈峰, 有机化学, 2018, 38,802.) |
| [15] | Magano, J.; Monfette, S. ACS Catal. 2015, 5,3120. |
| [16] | Tarui, A.; Shinohara, S.; Sato, K.; Omote, M.; Ando, A. Org. Lett. 2016, 18,1128. |
| [17] | (a) Matsubara, K.; Yamamoto, H.; Miyazaki, S.; Inatomi, T.; Nonaka, K.; Koga, Y.; Yamada, Y.; Veiros, L.F.; Kirchner, K. Organometallics, 2017, 36,255. |
| [17] | (b) Gan, Y.; Zhang, N.H.; Huang, S.X.; Liu, Y.H. Chin. J. Chem. 2020, 38,1686. |
| [17] | (c) Wang, H.; Wang, A.W.; Xia, Z.Z.; Zhou, W.Y.; Sun, Z.H.; Quan, J.F.; He, M.Y. Chin. J. Org. Chem. 2020, 40,2099. (in Chinese) |
| [17] | ( 王慧, 王安玮, 夏珍珍, 周维友, 孙中华, 钱俊峰, 何明阳, 有机化学, 2020, 40,2099.) |
| [17] | (d) He, S.J.; Pi, J.J.; Li, Y.; Lu, X.; Fu, Y. Acta Chim. Sinica 2018, 76,956. (in Chinese) |
| [17] | ( 何世江, 皮静静, 李炎, 陆熹, 傅尧, 化学学报, 2018, 76,956.) |
| [18] | He, F.; Wang, Z.X. Tetrahedron 2017, 73,4450. |
| [19] | Wang, D.Y, Kawahata, M.; Yang, Z.K.; Miyamoto, K.; Komagawa, S.; Yamaguchi, K.; Wang, C.; Uchiyama, M. Nat. Commun. 2016, 7,12937. |
| [20] | Blum, J.; Gelman, D.; Baidossi, W.; Shakh, E.; Rosenfeld, A.; Aizenshtat, Z.; Wassermann, B.C.; Frick, M.; Heymer, B.; Schutte, S.; Wernik, S.; Schumann, H. J. Org. Chem. 1997, 62,8681. |
| [21] | Moria, Y.; Shigenob, C.; Luo, Y.; Chan, B.; Onodera, G.; Kimura, M. Synlett 2018, 29,742. |
| [22] | Liu, C.Y.; Wititsuwannakul, T.; Hsieh, C.H.; Tsai, C.Y.; Wang, T.H.; Ambre, R.; Chen, W.C.; Surawatanawong, P.; Ong, T.G. J. Chin. Chem. Soc. 2020, 67,376. |
| [23] | Naganawa, Y.; Guo, H.Q.; Sakamoto, K.; Nakajima, Y. ChemCatChem 2019, 11,3756. |
| [24] | Sato, F.; Kodama, H.; Sato, M. J. Organomet. Chem. 1978, 157,C30. |
| [25] | Shrestha, B.; Thapa, S.; Gurung, S.K.; Pike, R.A. S.; Giri, R. J. Org. Chem. 2016, 81,787. |
| [26] | Ne?as, D.; Kotora, M.; Císa?ová, I. Eur. J. Org. Chem. 2004, 6,1280. |
| [27] | Ne?as, D.; Drabina, P.; Sedlák, M.; Kotora, M. Tetrahedron Lett. 2007, 48,4539. |
| [28] | Chen, Q.; Ilies, L.; Yoshikai, N.; Nakamura, E. Org. Lett. 2011, 13,3232. |
| [29] | Chen, X.; Li, J.; Hao, X.; Goodhue, C.E.; Yu, J.Q. J. Am. Chem. Soc. 2006, 128,78. |
| [30] | Dai, H.X.; Stepan, A.F.; Plummer, M.S.; Zhang, Y.-H.; Yu, J.Q. J. Am. Chem. Soc. 2011, 133,7222. |
| [31] | Ilies, L.; Matsubara, T.; Ichikawa, S.; Asako, S.; Nakamura, E. J. Am. Chem. Soc. 2014, 136,13126. |
| [32] | Zhang, S.Y.; He, G.; Nack, W.A.; Zhao, Y.; Li, Q.; Chen, G. J. Am. Chem. Soc. 2013, 135,2124. |
| [33] | Shang, R.; Ilies, L.; Nakamura, E. J. Am. Chem. Soc. 2015, 137,7660. |
| [34] | Shang, R.; Ilies, L.; Nakamura, E. J. Am. Chem. Soc. 2016, 138,10132. |
| [35] | Su, B.; Cao, Z.C.; Shi, Z.J. Acc. Chem. Res. 2015, 48,886. |
| [36] | Liu, W.; Groves, J.T. Acc. Chem. Res. 2015, 48,1727. |
| [37] | Tasker, S.Z.; Standley, E.A.; Jamison, T.F. Nature 2014, 509,299. |
| [38] | Moselage, M.; Li, J.; Ackermann, L. ACS Catal. 2016, 6,498. |
| [39] | Gao, K.; Yoshikai, N. Acc. Chem. Res. 2014, 47,1208. |
| [40] | Liang, Y.; Jiao, N. Angew. Chem., nt. Ed. 2016, 55,4035. |
| [41] | (a) Hummel, J.R.; Ellman, J.A. J. Am. Chem. Soc., 2015, 137,490. |
| [41] | Gu, Z.Y.; Ji, S.J. Acta Chim. Sinica 2018, 76,347. (in Chinese) |
| [41] | ( 顾正洋, 纪顺俊, 化学学报, 2018, 76,347.) |
| [42] | Chen, Q.; Ilies, L.; Nakamura, E. J. Am. Chem. Soc. 2011, 133,428. |
| [43] | Li, B.; Wu, Z.H.; Gu, Y.F.; Sun, C.L.; Wang, B.Q.; Shi, Z.J. Angew. Chem., nt. Ed. 2011, 50,1109. |
| [44] | Mei, R.H.; Ackermann, L. Adv. Synth. Catal. 2016, 358,2443. |
| [45] | Punji, B.; Song, W.F.; Shevchenko, G.A.; Ackermann, L. Chem.-Eur. J. 2013, 19,10605. |
| [46] | Gao, K.; Yoshikai, N. J. Am. Chem. Soc. 2013, 135,9279. |
| [47] | Wang, H.Q.; Zhang, S.; Wang, Z.Q.; He, M.H.; Xu, K. Org. Lett. 2016, 18,5628. |
| [48] | Xu, K.; Tan, Z.M.; Zhang, H.N.; Zhang, S. Synthesis 2017,3931. |
| [49] | Li, Q.; Li, Y.R.; Hu, W.P.; Hu, R.J.; Li, G.G.; Lu, H.J. Chem.-Eur. J. 2016, 22,12286. |
| [50] | Arisawa, M.; Torisawa, Y.; Nakagawa, M. Synthesis 1995,1371. |
| [51] | Arisawa, M.; Torisawa, Y.; Kawahara, M.; Yamanaka, M.; Nishida, A.; Nakagawa, M. J. Org. Chem., 1997, 62,4327. |
| [52] | (a) Amii, H.; Uneyama, K. Chem. Rev. 2009, 109,2119. |
| [52] | Ren, Z.W.; Ren, N.; Zhang, F.G.; Ma, J.A. Acta Chim. Sinica 2018, 76,940. (in Chinese) |
| [52] | ( 任智雯, 任楠, 张发光, 马军安, 化学学报, 2018, 76,940.) |
| [53] | Gu, W.X.; Haneline, M.R.; Douvris, C.; Ozerov, O.V. J. Am. Chem. Soc. 2009, 131,11203. |
| [54] | (a) Dong, D.Q.; Li, G.H.; Chen, D.M.; Sun, Y.Y.; Han, J.J.; Wang, Z.L.; Xu, X.M.; Yu, X.Y. Chin. J. Org. Chem. 2020, 40,1766. (in Chinese) |
| [54] | ( 董道青, 李光辉, 陈德茂, 孙媛媛, 韩晴晴, 王祖利, 徐鑫明, 于贤勇, 有机化学, 2020, 40,1766.) |
| [54] | (b) Li, Y.P.; Wang, M.; Jiang, X.F. Chin. J. Chem., 2020, 38,1521. |
| [55] | Hashimoto, S.; Kitagawa, Y.; Iemura, S.; Yamamote, H.; Nozaki, H. Tetrahedron Lett. 1976, 30,2615. |
| [56] | Ooi, T.; Uraguchi, D.; Kagoshima, N.; Maruoka, K. Tetrahedron Lett. 1997, 38,5679. |
| [57] | Terao, J.; Begum, S.A.; Shinohara, Y.; Tomita, M.; Naitoh, Y.; Kambe, N. Chem. Commun. 2007,855. |
| [58] | Terao, J.; Nakamura, M.; Kambe, N. Chem. Commun. 2009,6011. |
| [59] | Tanaka, H.; Shishido, Y. Bioorg. Med. Chem. Lett. 2007, 17,6079. |
| [60] | Shimada, H.; Kikuchi, S.; Haraguchi, K.; Tanaka, H. Carbohydr. Res. 2010, 345,2616. |
| [61] | Miyoshi, T.; Miyakawa, T.; Ueda, M.; Miyata, O. Angew. Chem., nt. Ed. 2011, 50,928. |
| [62] | Smith, D.A.; Banks, S.W. Phytochemistry 1986, 25,979. |
| [63] | Goel, A.; Kumar, A.; Raghuvanshi, A. Chem. Rev. 2013, 113,1614. |
| [64] | Feng, Z.G.; Bai, W.J.; Pettus, T.R. R. Angew. Chem., nt. Ed. 2015, 54,1864. |
| [65] | Nakamura, K.; Ohmori, K.; Suzuki, K. Chem. Commun. 2015, 51,7012. |
| [66] | (a) Shuai, Liu, S.; Li, J.; Wang, D.L.; Liu, F.; Liu, X.; Gao, Y.Y.; Jie, D.; Cheng, C. Chin. J. Chem. 2019, 37,570. |
| [66] | (b) Dong, K.; Liu, Q.; Wu, Li.Z. Acta Chim. Sinica 2020, 78,299. (in Chinese) |
| [66] | ( 董奎, 刘强, 吴骊珠, 化学学报, 2020, 78,299.) |
| [66] | (c) Ma, X.D.; Zhang, G.Z. Chin. J. Chem. 2020, 38,1299. |
| [66] | (d) Chen, Y.F.; Zhao, H.; Cheng, D.P.; Li, X.N.; Xu, X.L. Chin. J. Org. Chem. 2020, 40,1297. (in Chinese) |
| [66] | ( 陈跃峰, 赵赫, 程冬萍, 李小年, 许孝良, 有机化学, 2020, 40,1297.) |
/
| 〈 |
|
〉 |