

铜催化羧酸与芳氨基甲酰氯的脱羧交叉偶联
收稿日期: 2020-07-30
修回日期: 2020-10-20
网络出版日期: 2020-11-19
基金资助
中央高校基本科研业务费专项资金(2572019CG06); 中央高校基本科研业务费专项资金(2572020DR07); 黑龙江省自然科学基金(LC2018003); 黑龙江省自然科学基金(B2017002); 高等学校学科创新引智计划(111计划)(B20088)
Copper-Catalyzed Decarboxylative Cross-Coupling of Carboxylic Acids and Arylcarbamoyl Chlorides
Received date: 2020-07-30
Revised date: 2020-10-20
Online published: 2020-11-19
Supported by
Fundamental Research Funds for the Central Universities(2572019CG06); Fundamental Research Funds for the Central Universities(2572020DR07); Natural Science Foundation of Heilongjiang Province(LC2018003); Natural Science Foundation of Heilongjiang Province(B2017002); Programme of Introducing Talents of Discipline to Universities (111 Project)(B20088)
周敦 , 樊爱红 , 李翔 , 陈春霞 , 孙鹏 , 彭进松 . 铜催化羧酸与芳氨基甲酰氯的脱羧交叉偶联[J]. 有机化学, 2021 , 41(3) : 1146 -1152 . DOI: 10.6023/cjoc202007071
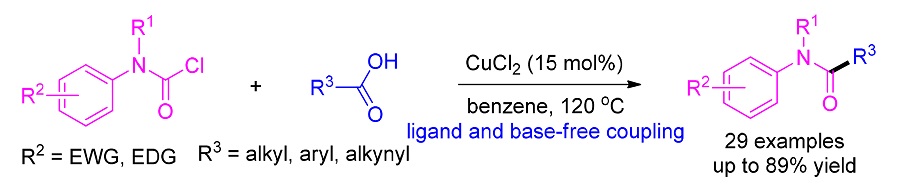
A ligand-free copper-catalyzed decarboxylative cross-coupling reaction of carboxylic acids and carbamoyl chlorides in the absence of base was developed. With CuCl2 as the catalyst, the decarboxylative cross-coupling process could be realized in benzene at 120 ℃ in 48 h. Under the standard condition, the catalytic system had good functional group tolerance, and diverse amides were obtained in good to high yields. The structures of products were elucidated by 1H NMR, 13C NMR and HRMS spectra.
| [1] | (a) Valeur, E.; Bradley, M. Chem. Soc. Rev. 2009, 38, 606. |
| [1] | (b) de Figueiredo, R.M.; Suppo,, J.-S.; Campagne,, J.-M. Chem. Rev. 2016, 116, 12029. |
| [2] | Roy, S.; Roy, S.; Gribble, G. W. Tetrahedron 2012, 68, 9867. |
| [3] | Organotin reagents, see: (a) Balas, L.; Jousseaume, B.; Shin, H.; Verlhac, J.-B.; Wallian, F. Organometallics 1991, 10, 366. |
| [3] | (b) Jousseaume, B.; Kwon, H.; Verlhac, J.-B.; Denat, F.; Dubac, J. Synlett 1993,117. |
| [3] | (c) Murakami, M.; Hoshino, Y.; Ito, H.; Ito, Y. Chem. Lett. 1998,163. |
| [3] | (d) Hu, W.; Zheng, J.; Li, M.; Wu, W.; Liu, H.; Jiang, H. Chin. J. Chem. 2018, 36, 712. |
| [4] | Lemoucheux, L.; Rouden, J.; Lasne, M.-C. Tetrahedron Lett. 2000, 41, 9997. |
| [5] | Rieke, R. D.; Kim, S.-H. Tetrahedron Lett. 2012, 53, 3478. |
| [6] | Lysén, M.; Kelleher, S.; Begtrup, M.; Kristensen, J. L. J. Org. Chem. 2005, 70, 5342. |
| [7] | Duan, Y.-Z.; Deng, M.-Z. Synlett 2005,355. |
| [8] | Yasui, Y.; Tsuchida, S.; Miyabe, H.; Takemoto, Y. J. Org. Chem. 2007, 72, 5898. |
| [9] | Krishnamoorthy, R.; Lam, S. Q.; Manley, C. M.; Herr, R. J. J. Org. Chem. 2010, 75, 1251. |
| [10] | Kochi, T.; Urano, S.; Seki, H.; Mizushima, E.; Sato, M.; Kakiuchi, F. J. Am. Chem. Soc. 2009, 131, 2792. |
| [11] | (a) Matsuzono, M.; Fukuda, T.; Iwao, M. Tetrahedron Lett. 2001, 42, 7621. |
| [11] | (b) Chao, W.-R.; Yean, D.; Amin, K.; Green, C.; Jong, L. J. Med. Chem. 2007, 50, 3412. |
| [12] | Lemoucheux, L.; Seitz, T.; Rouden, J.; Lasne, M.-C. Org. Lett. 2004, 6, 3703. |
| [13] | Selected reviews, see: (a) Baudoin, O. Angew. Chem., Int. Ed. 2007, 46, 1373. |
| [13] | (b) Gooβen, L. J.; Rodriguez, N.; Gooβen, K. Angew. Chem.. Int. Ed. 2008, 47, 3100. |
| [13] | (c) Rodríguez, N.; Gooβen, L. J. Chem. Soc. Rev. 2011, 40, 5030. |
| [13] | (d) Dzik, W. I.; Lange, P. P.; Gooβen, L. J. Chem. Sci. 2012, 3, 2671. |
| [13] | (e) Wei, Y.; Hu, P.; Zhang, M.; Su, W. Chem. Rev. 2017, 117, 8864. |
| [14] | Selected examples, see: (a) Myers, A. G.; Tanaka, D.; Mannion, M. R. J. Am. Chem. Soc. 2002, 124, 11250. |
| [14] | (b) Tanaka, D.; Romeril, S. P.; Myers, A. G. J. Am. Chem. Soc. 2005, 127, 10323. |
| [15] | Goo?en, L. J.; Deng, G.; Levy, L. M. Science 2006, 313, 662. |
| [16] | (a) Goo?en, L. J.; Linder, C.; Rodriguez, N.; Lange, P. P.; Fromm, A. Chem. Commun. 2009,7173. |
| [16] | (b) Cornella, J.; Sanchez, C.; Banawa, D.; Larrosa, I. Chem. Commun. 2009,7176. |
| [17] | (a) Cornella, J.; Rosillo-Lopez, M.; Larrosa, I. Adv. Synth. Catal. 2011, 353, 1359. |
| [17] | (b) Dupuy, S.; Lazreg, F.; Slawin, A. M. Z.; Cazin, C. S. J.; Nolan, S. P. Chem. Commun. 2011, 47, 5455. |
| [18] | Sun, Z.-M.; Zhao, P. Angew. Chem., Int. Ed. 2009, 48, 6726. |
| [19] | Shang, R.; Fu, Y.; Wang, Y.; Xu, Q.; Yu, H.-Z.; Liu, L. Angew. Chem., Int. Ed. 2009, 48, 9350. |
| [20] | (a) Jiao, J.; Zhang, X.-R.; Chang, N.-H.; Wang, J.; Wei, J.-F.; Shi, X.-Y.; Chen, Z.-G. J. Org. Chem. 2011, 76, 1180. |
| [20] | (b) Iwai, T.; Fujihara, T.; Terao, J.; Tsuji, Y. J. Am. Chem. Soc. 2010, 132, 9602. |
| [21] | Yamasaki, R.; Morita, K.; Iizumi, H.; Ito, A.; Fukuda, K.; Okamoto, I. Chem.-Eur. J. 2019, 25, 10118. |
| [22] | Allah, T. N.; ne Savourey, S.; Berthet, J.-C.; Nicolas, E.; Cantat, T. Angew. Chem., Int. Ed. 2019, 58, 10884. |
| [23] | Song, G.; Sun, G.; Tang, Y.; Mai, W. J. Chem. Res. 2013,630. |
| [24] | Zhou, Y.; Zhang, X.; Zhang, Y.; Ruan, L.; Zhang, J.; Zhang-Negrerie, D.; Du, Y. Org. Lett. 2017, 19, 150. |
| [25] | Zhang, Z.; Liu, Y.-H.; Zhang, X.; Wang, X.-C. Tetrahedron 2019, 75, 2763. |
| [26] | Bao, Y.-S.; Zhaorigetu, B.; Agula, B.; Baiyin, M.; Jia, M. J. Org. Chem. 2014, 79, 803. |
| [27] | Ackermann, L.; Vicente, R.; Hofmann, N. Org. Lett. 2009, 11, 4274. |
| [28] | Das, K. G.; Funke, P. T.; Bose, A. K. J. Am. Chem. Soc. 1964, 86, 3729. |
/
| 〈 |
|
〉 |