

过渡金属催化的环丙烯聚合反应研究
收稿日期: 2020-10-16
修回日期: 2020-11-08
网络出版日期: 2020-12-01
基金资助
国家自然科学基金(91956104); 北京市高等学校卓越青年科学家计划(BJJWZYJH01201910001001)
Transition-Metal-Catalyzed Polymerization of Cyclopropenes
Received date: 2020-10-16
Revised date: 2020-11-08
Online published: 2020-12-01
Supported by
National Natural Science Foundation of China(91956104); Beijing Outstanding Young Scientist Program(BJJWZYJH01201910001001)
张泽鹏 , 郜云鹏 , 陈树峰 , 王剑波 . 过渡金属催化的环丙烯聚合反应研究[J]. 有机化学, 2021 , 41(5) : 1888 -1896 . DOI: 10.6023/cjoc202010024
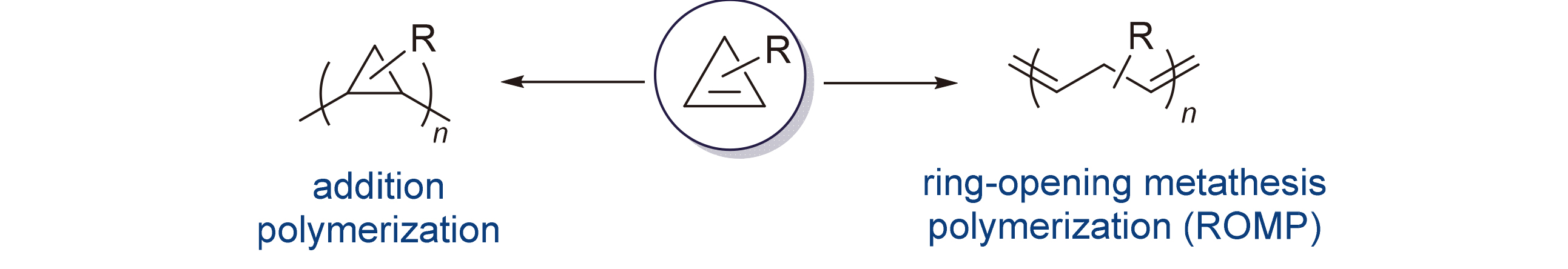
As the smallest unsaturated cyclic compounds in nature, cyclopropenes serve as important synthetic intermediates in organic chemistry as well as unique monomers in polymerization. While cyclopropenes are mostly explored in the domain of organic chemistry, the polymerization reaction of cyclopropenes has gradually attracted the attentions of chemists in recent years. The research progress of cyclopropene polymerization is reviewed, including addition polymerization and ring-opening metathesis polymerization (ROMP), and prospects for the future development of this field from the perspective of polymer synthesis methodolody.
| [1] | Stgrzel, M.; Mihan, S.; Mulhaupt, R. Chem. Rev. 2016, 116, 1398. |
| [2] | Chen, C. Nat. Rev. Chem. 2018, 2, 6. |
| [3] | Tan, C.; Chen, C. Angew. Chem., Int. Ed. 2019, 58, 7192. |
| [4] | Mu, H.; Pan, L.; Song, D.; Li, Y. Chem. Rev. 2015, 115, 12091. |
| [5] | Bermeshev, M. V.; Chapala, P. P. Prog. Polym. Sci. 2018, 84, 1. |
| [6] | Ma, S.; Cai, Y.; Tu, Y.; Guan, Y.; Chen, X. Polym. Chem. 2016, 7, 3520. |
| [7] | Rubin, M.; Rubina, M.; Gevorgyan, V. Chem. Rev. 2007, 107, 3117. |
| [8] | Zhu, Z.-B.; Wei, Y.; Shi, M. Chem. Soc. Rev. 2011, 40, 5534. |
| [9] | Vicente, R. Synthesis 2016, 48, 2343. |
| [10] | Vicente, R. Chem. Rev. 2021, 121, 162. |
| [11] | Zhang, H.; Wang, K.; Wang, B.; Yi, H.; Hu, F.; Li, C.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2014, 53, 13234. |
| [12] | Zhang, H.; Wang, B.; Wang, K.; Xie, G.; Li, C.; Zhang, Y.; Wang, J. Chem. Commun. 2014, 50, 8050. |
| [13] | Zhang, H.; Wang, B.; Yi, H.; Zhang, Y.; Wang, J. Org. Lett. 2015, 17, 3322. |
| [14] | Wang, B.; Yi, H.; Zhang, H.; Sun, T.; Zhang, Y.; Wang, J. J. Org. Chem. 2018, 83, 1026. |
| [15] | Dian, L. Y.; Marek, I. Chem. Rev. 2018, 118, 8415. |
| [16] | Dian, L. Y.; Marek, I. ACS Catal. 2020, 10, 1289. |
| [17] | Cheng, Q. Q.; Deng, Y. M.; Lankelma, M.; Doyle, M. P. Chem. Soc. Rev. 2017, 46, 5425. |
| [18] | Li, P.; Zhang, X.; Shi, M. Chem. Commun. 2020, 56, 5457. |
| [19] | Wiberg, K. B.; Bartley, W. J. J. Am. Chem. Soc. 1960, 82, 6375. |
| [20] | Weigert, F. J.; Baird, R. L.; Shapley, J. R. J. Am. Chem. Soc. 1970, 92, 6630. |
| [21] | Binger, P.; McMeeking, J.; Schuchardt, U. Chem. Ber. 1980, 113, 2372. |
| [22] | Binger, P.; Schuchardt, U. Chem. Ber. 1981, 114, 1649. |
| [23] | Binger, P.; Büch, H. M.; Benn, R.; Mynott, R. Angew. Chem., Int. Ed. Engl. 1982, 21, 62. |
| [24] | Rush, S.; Reinmuth, A.; Risse, W. J. Am. Chem. Soc. 1996, 118, 12230. |
| [25] | Rush, S.; Reinmuth, A.; Risse, W. Macromolecules 1997, 30, 7375. |
| [26] | Shintani, R.; Iino, R.; Nozaki, K. J. Am. Chem. Soc. 2014, 136, 7849. |
| [27] | Singh, R.; Czekelius, C. R.; Schrock, R. Macromolecules 2006, 39, 1316. |
| [28] | Meena, J. S.; Thankachan, P. P. Comput. Theor. Chem. 2013, 1024, 1. |
| [29] | Singh, R.; Schrock, R. R. Macromolecules 2008, 41, 2990. |
| [30] | Flook, M. M.; Gerber, L. C. H.; Debelouchina, G. T.; Schrock, R. R. Macromolecules 2010, 43, 7515. |
| [31] | Binder, W. H.; Kurzhals, S.; Pulamagatta, B.; Decker, U.; Pawar, G. M.; Wang, D.; Kühnel, C.; Buchmeiser, M. R. Macromolecules 2008, 41, 8405. |
| [32] | Binder, W. H.; Pulamagatta, B.; Kurzhals, O. K. S.; Barqawi, H.; Tanner, S. Macromolecules 2009, 42, 9457. |
| [33] | Dumas, A.; Tarrieu, R.; Vives, T.; Roisnel, T.; Dorcet, V.; Basle?, O.; Mauduit, M. ACS Catal. 2018, 8, 3257. |
| [34] | Peng, J.-J.; Panda, B.; Satyanarayana, K.; Yang, H.-R.; Huang, S.-L.; Huang, M. J.; Chen, C.-h.; Lai, G.; Lai, Y.-Y.; Luh, T.-Y. Macromolecules 2019, 52, 7749. |
| [35] | Elling, B. R.; Su, J. K.; Xia, Y. Chem. Commun. 2016, 52, 9097. |
| [36] | Elling, B. R.; Xia, Y. J. Am. Chem. Soc. 2015, 137, 9922. |
| [37] | Elling, B. R.; Xia, Y. ACS Macro Lett. 2018, 7, 656. |
| [38] | Elling, B. R.; Su, J. K.; Xia, Y. ACS Macro Lett. 2020, 9, 180. |
| [39] | Elling, B. R.; Su, J. K.; Feist, J. D.; Xia, Y. Chem 2019, 5, 2691. |
| [40] | Su, J. K.; Jin, Z.; Xia, Y. Angew. Chem., Int. Ed. 2019, 58, 17771. |
| [41] | Su, J. K.; Lee, S. Y.; Elling, B. R.; Xia, Y. Macromolecules 2020, 53, 5833. |
| [42] | For a recent example of Grubbs-type cis-selective living ROMP, see: Song, J. A.; Peterson, G. I.; Bang, K. T.; Ahmed, T. S.; Sung, J. C.; Grubbs, R. H.; Choi, T. L. J. Am. Chem. Soc. 2020, 142, 10438. |
/
| 〈 |
|
〉 |