

基于ABBV-075的新型溴结构域蛋白4 (BRD4)小分子抑制剂的设计、合成及活性评价
收稿日期: 2020-07-26
修回日期: 2020-10-21
网络出版日期: 2020-12-05
基金资助
国家自然科学基金(81438005); 国家自然科学基金(81773562); 国家蛋白质重点研究项目基金(SQ2018YFE011359); 河南省重点研究项目基金(1611003110100)
Design, Synthesis and Activity Evaluation of Novel Bromodomain-Containing Protein 4 (BRD4) Small Molecule Inhibitor Based on ABBV-075
Received date: 2020-07-26
Revised date: 2020-10-21
Online published: 2020-12-05
Supported by
National Natural Science Foundation of China(81438005); National Natural Science Foundation of China(81773562); National Key Research Program of Proteins(SQ2018YFE011359); Key Research Program of Henan Province(1611003110100)
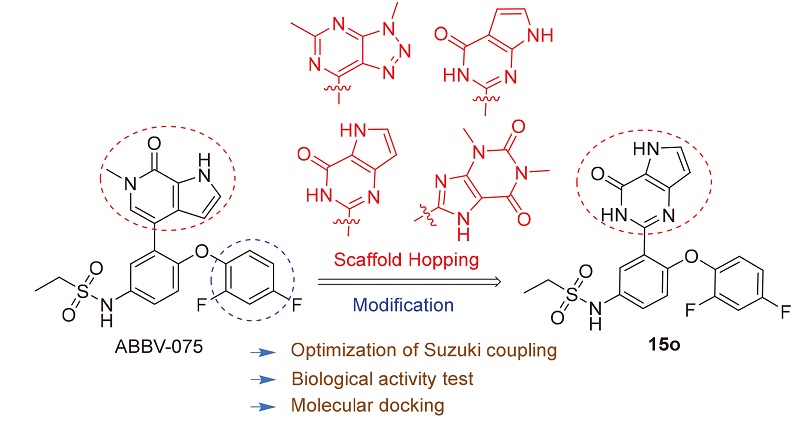
为发现新型溴结构域蛋白4 (BRD4)小分子抑制剂, 基于ABBV-075, 通过骨架跃迁, 设计并合成4种不同母核, 共16个化合物. 优化了合成步骤中Suzuki偶联反应条件, 并测试了化合物对BRD4的抑制活性. 结果表明, N-(4-(2,4-二氟氧代苯基)-3-(4-氧代-4,5-二氢-3H-吡咯并[2,3-d]嘧啶-2-基)苯基)乙磺酰胺(15o)的抑制率在10 μmol/L达到了51%, IC 50=(16.39±1.20) μmol/L, 具有明显的BRD4抑制活性. 分子对接结果表明,15o可与Asn433和Asp381形成关键氢键, 其与ABBV-075同源物的叠合图显示了二者结合模式的差异. 阐明了化合物15o与ABBV-075活性差异的原因, 为进一步发现高活性BRD4抑制剂提供设计思路.
徐晨淏 , 龚云鹏 , 陈雅欣 , 宋启梦 , 李娇 , 郑一超 , 李雯 , 孙凯 , 刘宏民 . 基于ABBV-075的新型溴结构域蛋白4 (BRD4)小分子抑制剂的设计、合成及活性评价[J]. 有机化学, 2021 , 41(4) : 1712 -1721 . DOI: 10.6023/cjoc202007059
In order to discover novel bromodomain-containing protein 4 (BRD4) small molecule inhibitor, 16 compounds with 4 different nuclei based on ABBV-075 were designed and synthesized through scaffold hopping. The conditions of the Suzuki coupling reaction in the synthesis steps were optimized and the activity of all compounds against BRD4 was tested. The results indicated that compound N-(4-(2,4-difluorophenoxy)-3-(4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidin-2-yl)- phenyl)ethanesulfonamide (15o) had 51% at concentration of 10 μmol/L with IC 50 value of (16.39±1.20) μmol/L, possessing obvious BRD4 inhibitory activity. The molecular docking results showed that15oformed key hydrogen bonds with Asn433 and Asp381. The picture of superposed conformation of 15o and homolog of ABBV-075 exhibited the combination difference between them, explaining the reason of activity gap, which would provide excellent ideas for further study.

Key words: BRD4 inhibitor; ABBV-075; synthesis and optimization; bioactivity; molecular docking
| [1] | Smith, S.G.; Zhou, M.-M. ACS Chem. Biol. 2016, 11,598. |
| [2] | Filippakopoulos, P.; Picaud, S.; Mangos, M.; Keates, T.; Lambert, J.-P.; Barsyte-Lovejoy, D.; Felletar, I.; Volkmer, R.; Müller, S.; Pawson, T.; Gingras, A.-C.; Arrowsmith, C.H.; Knapp, S. Cell 2012, 149,214. |
| [3] | Lee, J.-E.; Park, Y.-K.; Park, S.; Jang, Y.; Waring, N.; Dey, A.; Ozato, K.; Lai, B.; Peng, W.; Ge, K. Nat. Commun. 2017, 8,2217. |
| [4] | Muller, S.; Filippakopoulos, P.; Knapp, S. Expert. Rev. Mol.Med. 2011, 13,e29. |
| [5] | Qin, Z.-Y.; Wang, T.; Su, S.; Shen, L.-T.; Zhu, G.-X.; Liu, Q.; Zhang, L.; Liu, K.-W.; Zhang, Y.; Zhou, Z.-H.; Zhang, X.-N.; Wen, L.-Z.; Yao, Y.-L.; Sun, W.-J.; Guo, Y.; Liu, K.-J.; Liu, L.; Wang, X.-W.; Wei, Y.-L.; Wang, J.; Xiao, H.-L.; Liu, P.; Bian, X.-W.; Chen, D.-F.; Wang, B. Cancer Res. 2019, 79,4869. |
| [6] | Wang, Q.; Sun, Y.; Li, T.; Liu, L.; Zhao, Y.; Li, L.; Zhang, L.; Meng, Y. Mol. Med. Rep. 2019, 19,499. |
| [7] | Ghosh, S.; Lora, J.M. Drug Discovery Today: Technol. 2016, 19,39. |
| [8] | Hargreaves, D.C.; Horng, T.; Medzhitov, R. Cell 2009, 138,129. |
| [9] | Liu, Z.; Wang, P.; Chen, H.; Wold, E.A.; Tian, B.; Brasier, A.R.; Zhou, J. J. Med. Chem. 2017, 60,4533. |
| [10] | Duan, Y.; Guan, Y.; Qin, W.; Zhai, X.; Yu, B.I. N.; Liu, H. MedChemComm 2019,10. |
| [11] | Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W.B.; Fedorov, O.; Morse, E.M.; Keates, T.; Hickman, T.T.; Felletar, I.; Philpott, M.; Munro, S.; McKeown, M.R.; Wang, Y.; Christie, A.L.; West, N.; Cameron, M.J.; Schwartz, B.; Heightman, T.D.; La Thangue, N.; French, C.A.; Wiest, O.; Kung, A.L.; Knapp, S.; Bradner, J.E. Nature 2010, 468,1067. |
| [12] | Dawson, M.A.; Prinjha, R.K.; Dittmann, A.; Giotopoulos, G.; Bantscheff, M.; Chan, W.-I.; Robson, S.C.; Chung, C.-w.; Hopf, C.; Savitski, M.M.; Huthmacher, C.; Gudgin, E.; Lugo, D.; Beinke, S.; Chapman, T.D.; Roberts, E.J.; Soden, P.E.; Auger, K.R.; Mirguet, O.; Doehner, K.; Delwel, R.; Burnett, A.K.; Jeffrey, P.; Drewes, G.; Lee, K.; Huntly, B.J. P.; Kouzarides, T. Nature 2011, 478,529. |
| [13] | Knapp, S.; Arruda, P.; Blagg, J.; Burley, S.; Drewry, D.H.; Edwards, A.; Fabbro, D.; Gillespie, P.; Gray, N.S.; Kuster, B.; Lackey, K.E.; Mazzafera, P.; Tomkinson, N.C. O.; Willson, T.M.; Workman, P.; Zuercher, W.J. Nat. Chem. Biol. 2013, 9,3. |
| [14] | Ember, S.W. J.; Zhu, J.-Y.; Olesen, S.H.; Martin, M.P.; Becker, A.; Berndt, N.; Georg, G.I.; Sch?nbrunn, E. ACS Chem. Biol. 2014, 9,1160. |
| [15] | Liu, S.; Yosief, H.O.; Dai, L.; Huang, H.; Dhawan, G.; Zhang, X.; Muthengi, A.M.; Roberts, J.; Buckley, D.L.; Perry, J.A.; Wu, L.; Bradner, J.E.; Qi, J.; Zhang, W. J. Med. Chem. 2018, 61,7785. |
| [16] | McDaniel, K.F.; Wang, L.; Soltwedel, T.; Fidanze, S.D.; Hasvold, L.A.; Liu, D.; Mantei, R.A.; Pratt, J.K.; Sheppard, G.S.; Bui, M.H.; Faivre, E.J.; Huang, X.; Li, L.; Lin, X.; Wang, R.; Warder, S.E.; Wilcox, D.; Albert, D.H.; Magoc, T.J.; Rajaraman, G.; Park, C.H.; Hutchins, C.W.; Shen, J.J.; Edalji, R.P.; Sun, C.C.; Martin, R.; Gao, W.; Wong, S.; Fang, G.; Elmore, S.W.; Shen, Y.; Kati, W.M. J. Med. Chem. 2017, 60,8369. |
| [17] | Faivre, E.J.; Wilcox, D.; Lin, X.; Hessler, P.; Torrent, M.; He, W.; Uziel, T.; Albert, D.H.; McDaniel, K.; Kati, W.; Shen, Y. Mol. Cancer Res. 2017, 15,35. |
| [18] | Li, Z.; Xiao, S.; Yang, Y.; Chen, C.; Lu, T.; Chen, Z.; Jiang, H.; Chen, S.; Luo, C.; Zhou, B. J. Med. Chem. 2020, 63,3956. |
| [19] | Sheppard, G.S.; Wang, L.; Fidanze, S.D.; Hasvold, L.A.; Liu, D.; Pratt, J.K.; Park, C.H.; Longenecker, K.; Qiu, W.; Torrent, M.; Kovar, P.J.; Bui, M.; Faivre, E.; Huang, X.; Lin, X.; Wilcox, D.; Zhang, L.; Shen, Y.; Albert, D.H.; Magoc, T.J.; Rajaraman, G.; Kati, W.M.; McDaniel, K.F. J. Med. Chem. 2020, 63,5585. |
| [20] | Brown, N. Mol. Inf. 2014, 33,458. |
| [21] | Li, Z.-H.; Yang, D.-X.; Geng, P.-F.; Zhang, J.; Wei, H.-M.; Hu, B.; Guo, Q.; Zhang, X.-H.; Guo, W.-G.; Zhao, B.; Yu, B.; Ma, L.-Y.; Liu, H.-M. Eur. J. Med. Chem. 2016, 124,967. |
| [22] | Xie, H.; Zeng, L.; Zeng, S.; Lu, X.; Zhang, G.; Zhao, X.; Cheng, N.; Tu, Z.; Li, Z.; Xu, H.; Yang, L.; Zhang, X.; Huang, M.; Zhao, J.; Hu, W. Eur. J. Med. Chem. 2012, 52,205. |
| [23] | Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95,2457. |
| [24] | Ramgren, S.D.; Hie, L.; Ye, Y.; Garg, N.K. Org. Lett. 2013, 15,3950. |
| [25] | Hu, Y.-H. WO 2018086585, 2018. |
| [26] | Hu, Y.-H. WO 2018086604, 2018. |
/
| 〈 |
|
〉 |