

氮杂环卡宾(NHC)/银(I)共催化合成2-氧代-2-芳乙基芳甲酸酯
收稿日期: 2020-08-20
修回日期: 2020-10-13
网络出版日期: 2020-12-24
基金资助
国家自然科学基金(2177020721); 国家自然科学基金(21871113); 江苏高等学校自然科学基金(17KJA150003)
N-Heterocyclic Carbene (NHC)/Ag(I) Co-catalyzed Synthesis of 2-Oxo-2-arylethyl Aryl Formates
Received date: 2020-08-20
Revised date: 2020-10-13
Online published: 2020-12-24
Supported by
National Science Foundation of China(2177020721); National Science Foundation of China(21871113); Natural Science Foundation of the Jiangsu Higher Education Institution(17KJA150003)
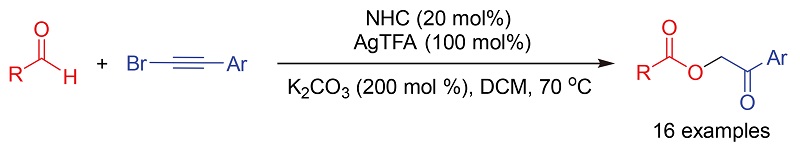
在氮杂环卡宾(NHC)/银(I)的共催化下, 以芳醛和(溴乙炔)苯及其衍生物为原料实现了2-氧代-2-芳乙基芳甲酸酯高效合成. 该方法具有底物范围广、原料简单易得、操作简便等优点, 为α-酰氧基羰基衍生物的简捷合成提供了新思路.
关键词: 氮杂环卡宾(N-heterocyclic carbene; NHC); 银(I); 共催化; α-酰氧基酮
成立 , 王文蓉 , 孙玉倩 , 李团结 , 于晨侠 , 姚昌盛 . 氮杂环卡宾(NHC)/银(I)共催化合成2-氧代-2-芳乙基芳甲酸酯[J]. 有机化学, 2021 , 41(4) : 1607 -1613 . DOI: 10.6023/cjoc202008035
An N-heterocyclic carbene (NHC)/Ag(I) co-catayzed efficient synthesis of 2-oxo-2-arylethyl aryl formates was realized by the reaction of aryl aldehydes with (bromoethynyl)benzenes. This method features broad substrate scope, ready availability of starting materials and operational simplicity, which gives an alternative access to α-acyloxycarbonyl derivatives.

| [1] | Lin, L.; Mulholland, N.; Wu, Q.-Y.; Beattie, D.; Huang, S.-W.; Irwin, D.; Clough, J.; Gu, Y.-C.; Yang, G.-F. J. Agric. Food Chem. 2012, 60,4480. |
| [2] | Wang, X.; Sena Filho, J.G.; Hoover, A.R.; King, J.B.; Ellis, T.K.; Powell, D.R.; Cichewicz, R.H. J. Nat. Prod. 2010, 73,942. |
| [3] | Wang, H.; Wang, Y.; Wang, W.; Fu, P.; Liu, P.; Zhu, W. J. Nat. Prod. 2011, 74,2014. |
| [4] | (a) Sabbah, D.A.; Saada, M.; Khalaf, R.A.; Bardaweel, S.; Sweidan, K.; Al-Qirim, T.; Al-Zughier, A.; Halim, H.A.; Sheikh, G.A. Bioorg. Med. Chem. Lett. 2015, 25,3120. |
| [4] | (b) Che, Y.; Wen, D.; Huang, Z.; Huang, M.; Luo, Y.; Liu, B.; Lu, H.; Wu, Y.; Peng, Y.; Zhang, J. Bioorg. Med. Chem. Lett. 2012, 22,6867. |
| [5] | Dai, L.; Yu, S.; Xiong, W.; Chen, Z.; Xu, T.; Shao, Y.; Chen, J. Adv. Synth. Catal. 2020, 362,1893. |
| [6] | Kim, S.H.; Jang, M.; Moon, D.Y.; Park, B.S. Tetrahedron Lett. 2018, 59,4245. |
| [7] | Arai, M.A.; Kofuji, Y.; Tanaka, Y.; Yanase, N.; Yamaku, K.; Fuentes, R.G.; Karmakar, U.K.; Ishibashi, M. Org. Biomol. Chem. 2016, 14,3061. |
| [8] | (a) Funk, P.; Motyka, K.; D?ubák, P.; Znojek, P.; Gurská, S.; Kusz, J.; McMaster, C.; Hajdúch, M.; Soural, M. RSC Adv. 2015, 5,48861. |
| [8] | (b) Kadri?, J.; Motyka, K.; D?ubák, P.; Hajdúch, M.; Soural, M. Tetrahedron Lett. 2014, 55,3592. |
| [9] | (a) Prasad, P.K.; Reddi, R.N.; Arumugam, S. Org. Biomol. Chem. 2018, 16,9334. |
| [9] | (b) Zhou, X.; Ma, H.; Cao, J.; Liu, X.; Huang, G.; Org. Biomol. Chem. 2016, 14,10070. |
| [9] | (c) Zhu, Y.; Zheng, Y.; Song, W.; Wei, B.; Xuan, L. Tetrahedron Lett. 2018, 59,368. |
| [9] | (d) Zhu, M.; Wei, W.; Yang, D.; Cui, H.; Sun, X.; Wang, H. Org. Biomol. Chem. 2016, 14,10998. |
| [10] | (a) Kreibich, M.; Gemander, M.; Peter, D.; Yadav, D.; Koning, C.; Fernandes, M.; Green, I.; van Otterlo, W.; Brückner, R. Eur. J. Org. Chem. 2020, 19,2929. |
| [10] | (b) Liu, L.; Feng, S.; Li, C. ACS Sustainable Chem. Eng. 2016, 4,6754. |
| [10] | (c) Hu, Y.; Chen, J.; Le, Z.G.; Chen, Z.C.; Zheng, Q.G. Chin. Chem. Lett. 2005, 16,903. |
| [10] | (d) Wang, X.; Li, G.; Yang, Y.; Jiang, J.; Feng, Z.; Zhang, P. Chin. Chem. Lett. 2020, 31,711. |
| [11] | Mondal, B.; Sahoo, S.C.; Pan, S.C. Eur. J. Org. Chem. 2015, 14,3135. |
| [12] | Wang, J.-L.; Wang, J.-Q.; He, L.-N.; Dou, X.-Y.; Wu, F. Green Chem. 2008, 10,1218. |
| [13] | Ji, K.; Zhao, Y.; Zhang, L. Angew. Chem. Int. Ed. 2013, 52,6508. |
| [14] | Mu, Y.; Chen, Y.; Gao, Y.; Sun, J.; Iqbal, Z.; Wan, Y.; Yang, M.; Yang, Z.; Tang, D. ChemistrySelect 2020, 5,1705. |
| [15] | Tian, L.; Guo, Y.; Wei, L.; Wan, J.-P.; Sheng, S. Asian J. Org. Chem. 2019, 8,1484. |
| [16] | Li, J.; Yang, Z.; Yang, T.; Yi, J.; Zhou, C. New J. Chem. 2018, 42,1581. |
| [17] | Chen, C.; Liu, W.; Zhou, P.; Liu, H. RSC Adv. 2017, 7,20394. |
| [18] | Tan, L.; Chen, C.; Liu, W. Beilstein J. Org. Chem. 2017, 13,1079. |
| [19] | (a) Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Chem. Rev. 2015, 115,9307. |
| [19] | (b) Enders, D.; Niemeier, O.; Henseler, A. Chem. Rev. 2007, 107,5606. |
| [19] | (c) Wang, Z.; Li, R.; Qian, H.; Yao, C. Chin. J. Org. Chem. 2019, 39,2075. (in Chinese) |
| [19] | ( 王占林, 李如一, 钱辉旻, 姚昌盛, 有机化学, 2019, 39,2075.) |
| [19] | (d) Yao, C.; Xiao, Z.; Liu, R.; Li, T.; Jiao, W.; Yu, C. Chem. Eur. J. 2013, 19,456. |
| [19] | (e) Li, S.; Yang, W.; Luo, X.; Yao, C. Chin. J. Org. Chem. 2019, 39,1404. (in Chinese) |
| [19] | ( 李莎, 杨雯涵, 罗鲜, 姚昌盛, 有机化学, 2019, 39,1404.) |
| [19] | (f) Li, S.; Xu, J.; Luo, X.; Yang, W.; Yao, C. Chin. J. Org. Chem. 2020, 40,470. (in Chinese) |
| [19] | ( 李莎, 徐嘉煜, 罗鲜, 杨雯涵, 姚昌盛, 有机化学, 2020, 40,470.) |
| [19] | (g) Zhang, Y.; Xing, F.; Feng, Z.; Du, G.; Gu, C.; He, L. Chin. J. Org. Chem. 2020, 40,1608. (in Chinese) |
| [19] | ( 张阳, 邢芬, 冯泽男, 杜广芬, 顾承志, 何林, 有机化学, 2020, 40,1608.) |
| [19] | (h) Wang, A.; Xiao, Y.; Zhou, Y.; Xu, J.; Liu, H. Chin. J. Org. Chem. 2017, 37,2590. (in Chinese) |
| [19] | ( 王翱, 肖永龙, 周宇, 徐进宜, 柳红, 有机化学, 2017, 37,2590.) |
| [19] | (i) Qu, M.; He, J. Chin. J. Org. Chem. 2011, 31,1388. (in Chinese) |
| [19] | ( 屈孟男, 何金梅, 有机化学, 2011, 31,1388.) |
| [20] | Reddi, R.N.; Malekar, P.V.; Sudalai, A. Org. Biomol. Chem. 2013, 11,6477. |
| [21] | Reddi, R.N.; Gontala, A.; Prasad, P.K.; Sudalai, A. Asian J. Org. Chem. 2016, 5,48. |
| [22] | Forte, G.; Chiarotto, I.; Inesi, A.; Loreto, M.A.; Feroci, M. Adv. Synth. Catal. 2014, 356,1773. |
| [23] | Lu, H.; Liu, J.-Y.; Li, H.-Y.; Xu, P.-F. Acta Chim. Sinica 2018, 76,831. (in Chinese) |
| [23] | ( 鲁鸿, 刘金宇, 李红玉, 许鹏飞, 化学学报, 2018, 76,831.) |
| [24] | Nemoto, T.; Fukuda, T.; Hamada, Y. Tetrahedron Lett. 2006, 47,4365. |
| [25] | (a) Namitharan, K.; Zhu, T.; Cheng, J.; Zheng, P.; Li, X.; Yang, S.; Song, B.-A.; Chi, Y.R. Nat. Commun. 2014, 5,3982. |
| [25] | (b) Chen, J.; Yuan, P.; Wang, L.; Huang, Y. J. Am. Chem. Soc. 2017, 139,7045. |
| [26] | DiRocco, D.A.; Rovis, T. J. Am. Chem. Soc. 2012, 134,8094. |
| [27] | Chen, Z.-W.; Ye, D.-N.; Ye, M.; Zhou, Z.-G.; Li, S.-H.; Liu, L.-X. Tetrahedron Lett. 2014, 55,1373. |
| [28] | Jia, Y.; Li, T.; Yu, C.; Jiang, B.; Yao, C. Org. Biomol. Chem. 2016, 14,1982. |
| [29] | (a) Liu, Y.K.; Li, R.; Yue, L.; Li, B.J.; Chen, Y.C.; Wu, Y.; Ding, L.S. Org. Lett. 2006, 8,1521. |
| [29] | (b) Xia, Z.-H.; Dai, L.; Gao, Z.-H.; Ye, S. Chem. Commun. 2020, 56,1525. |
| [29] | (c) Gao, Z.-H.; Xia, Z.-H.; Dai, L.; Ye, S. Adv. Synth. Catal. 2020, 362,1819. |
| [30] | Liao, L.; Zhang, H.; Zhao, X. ACS Catal. 2018, 8,6745. |
| [31] | Hu, Y.; Chen, J.; Le, Z.G.; Chen, Z.C.; Zheng, Q.G. Chin. Chem. Lett. 2005, 16,903. |
/
| 〈 |
|
〉 |