

三乙胺促进的环丙烯酮和α-卤代异羟肟酸酯的环化反应合成多取代6H-1,3-噁嗪-6-酮
收稿日期: 2020-10-30
修回日期: 2020-12-10
网络出版日期: 2020-12-24
基金资助
国家自然科学基金(21472077); 国家自然科学基金(21772071); 广东省教育厅(2017KTSCX185); 广东省教育厅(2017KSYS010); 广东省教育厅(2019KZDXM035)
Triethyl Amine-Promoted Cyclization Reaction between Cyclopropenone and α-Halogenated Hydroxamate for the Synthesis of Polysubstituted 6H-1,3-Oxazin-6-one
Received date: 2020-10-30
Revised date: 2020-12-10
Online published: 2020-12-24
Supported by
National Natural Science Foundation of China(21472077); National Natural Science Foundation of China(21772071); Department of Education of Guangdong Province(2017KTSCX185); Department of Education of Guangdong Province(2017KSYS010); Department of Education of Guangdong Province(2019KZDXM035)
刘思展 , 崔明月 , 王博文 , 胡春梅 , 郑莹莹 , 李晶 , 徐学涛 , 王震 , 王少华 . 三乙胺促进的环丙烯酮和α-卤代异羟肟酸酯的环化反应合成多取代6H-1,3-噁嗪-6-酮[J]. 有机化学, 2021 , 41(4) : 1622 -1630 . DOI: 10.6023/cjoc202010041
A triethyl amine-promoted cyclization reaction between cyclopropenone and α-halohydroxamate has been developed to give an alternative synthetic strategy for the construction of 6H-1,3-oxazin-6-one skeleton. The reaction shows good yield and functional group tolerance under metal-free and mild conditions, and it is suitable for gram-scale preparation.
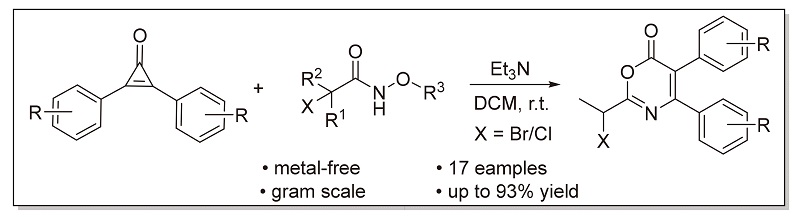
Key words: cyclopropenone; α-halogenated hydroxamate; cyclization reaction; oxazinone
| [1] | (a) Kopelman, P.; Bryson, A.; Hickling, R.; Rissanen, A.; Rossner, S.; Toubro, S.; Valensi, P. Int. J. Obes. 2007, 31,494. |
| [1] | (b) Yamada, Y.; Kato, T.; Ogino, H.; Ashina, S.; Kato, K. Horm. Metab. Res. 2008, 40,539. |
| [2] | Stein, R.L.; Strimpler, A.M.; Viscarello, B.R.; Wildonger, R.A.; Mauger, R.C.; Trainor, D.A. Biochemistry 1987, 26,4126. |
| [3] | Hays, S.J.; Caprathe, B.W.; Gilmore, J.L.; Amin, N.; Emmerling, M.R.; Michael, W.; Nadimpalli, R.; Nath, R.; Raser, K.J.; Stafford, D.; Watson, D.; Wang, K.; Jaen, J.C. J. Med. Chem. 1998, 41 1060. |
| [4] | Fenton, G.; Newton, C.G.; Wyman, B.M.; Bagge, P.; Dron, D.I.; Riddell, D.; Jones, G.D. J. Med. Chem. 1989, 32,265. |
| [5] | Jarvest, R.L.; Parratt, M.J.; Debouck, C.M.; Gorniak, J.G.; John Jennings, L.; Serafinowska, H.T.; Strickler, J.E. Bioorg. Med. Chem. Lett. 1996, 6,2463. |
| [6] | (a) Wu, X.-F.; Schranck, J.; Neumann, H.; Beller, M. Chem.-Eur. J. 2011, 17,12246. |
| [6] | (b) Liu, Q.; Chen, P.; Liu, G. ACS Catal. 2013, 3,178. |
| [6] | (c) Li, W.; Wu, X.-F. J. Org. Chem. 2014, 79,10410. |
| [6] | (d) Zhang, C.; Li, S.; Bure?, F.; Lee, R.; Ye, X.; Jiang, Z. ACS Catal. 2016, 6,6853. |
| [6] | (e) Song, P.; Yu, P.; Lin, J.-S.; Li, Y.; Yang, N.-Y.; Liu, X.-Y. Org. Lett. 2017, 19,1330. |
| [6] | (f) Niu, B.; Jiang, B.; Yu, L.-Z.; Shi, M. Org. Chem. Front. 2018, 5,1267. |
| [6] | (g) Mohan, R.D.; Jose, A. Asian J. Chem. 2018, 30,1075. |
| [6] | (h) Matsuda, T.; Yamanaka, K.; Tabata, Y.; Shiomi, T. Tetrahedron Lett. 2018, 59,1458. |
| [7] | Chen, M.; Ren, Z.H.; Wang, Y.Y.; Guan, Z.H. Angew. Chem., Int. Ed. 2013, 52,14196. |
| [8] | Karad, S.N.; Chung, W.K.; Liu, R.S. Chem. Sci. 2015, 6,5964. |
| [9] | Jurberg, I.D.; Davies, H.M. L. Org. Lett. 2017, 19,5158. |
| [10] | Lang, M.; Wang, J. Org. Chem. Front. 2019, 6,1367. |
| [11] | Breslow, R.; Haynie, R.; Mirra, J. J. Am. Chem. Soc. 1959, 81,247. |
| [12] | (a) Komatsu, K.; Kitagawa, T. Chem. Rev. 2003, 103,1371. |
| [12] | (b) Potts, K.T.; Baum, J.S. Chem. Rev. 1974, 74,189. |
| [12] | (c) Prasad, Raiguru, B.; Nayak, S.; Ranjan, Mishra, D.; Das, T.; Mohapatra, S.; Priyadarsini, Mishra, N. Asian J. Org. Chem. 2020, 9,1088. |
| [13] | (a) Matsumoto, K.; Ikemi, Y.; Hashimoto, S.; Lee, H.S.; Okamoto, Y. J. Org. Chem. 1986, 51,3729. |
| [13] | (b) Jacobs, C.A.; Dailey, W.P. J. Org. Chem. 1995, 60,7747. |
| [13] | (c) Cordes, M.H. J.; de Gala, S.; Berson, J.A. J. Am. Chem. Soc. 1994, 116,11161. |
| [14] | (a) K?rner, O.; Gleiter, R.; Rominger, F. Synthesis 2009,3259. |
| [14] | (b) Rivero, A.R.; Fernández, I.; Ramírez de Arellano, C.; Sierra, M.A. J. Org. Chem. 2015, 80,1207. |
| [14] | (c) Wallbaum, J.; Jones, P.G.; Werz, D.B. J. Org. Chem. 2015, 80,3730. |
| [14] | (d) Li, L.-H.; Jiang, Y.; Hao, J.; Wei, Y.; Shi, M. Adv. Synth. Catal. 2017, 359,3304. |
| [15] | (a) Matsuda, T.; Sakurai, Y. Eur. J. Org. Chem. 2013, 2013,4219. |
| [15] | (b) Yu, S.; Li, X. Org. Lett. 2014, 16,1220. |
| [15] | (c) Matsuda, T.; Sakurai, Y. Org. Chem. 2014, 79,2739. |
| [15] | (d) Xie, F.; Yu, S.; Qi, Z.; Li, X. Angew. Chem., Int. Ed. 2016, 55,15351. |
| [15] | (e) Kong, L.; Zhou, X.; Xu, Y.; Li, X. Org. Lett. 2017, 19,3644. |
| [15] | (f) Li, X.; Han, C.; Yao, H.; Lin, A. Org. Lett. 2017, 19,778. |
| [15] | (g) Ren, J.-T.; Wang, J.-X.; Tian, H.; Xu, J.-L.; Hu, H.; Aslam, M.; Sun, M. Org. Lett. 2018, 20,6636. |
| [15] | (h) Shan, L.; Wu, G.; Liu, M.; Gao, W.; Ding, J.; Huang, X.; Wu, H. Org. Chem. Front. 2018, 5,1651. |
| [15] | (i) Liu, Y.; Tian, Y.; Su, K.; Wang, P.; Guo, X.; Chen, B. Org. Chem. Front. 2019, 6,3973. |
| [16] | (a) Kondo, T.; Taniguchi, R.; Kimura, Y. Synlett 2018, 29,717. |
| [16] | (b) Haito, A.; Chatani, N. Chem. Commun. 2019, 55,5740. |
| [16] | (c) Bai, D.; Yu, Y.; Guo, H.; Chang, J.; Li, X. Angew. Chem., Int. Ed. 2020, 59,2740. |
| [16] | (d) Nanda, T.; Ravikumar, P.C. Org. Lett. 2020, 22,1368. |
| [16] | (e) Xing, H.; Chen, J.; Shi, Y.; Huang, T.; Hai, L.; Wu, Y. Org. Chem. Front. 2020, 7,672. |
| [16] | (f) Zhou, P.; Yang, W.-T.; Rahman, A.U.; Li, G.; Jiang, B. J. Org. Chem. 2020, 85,360. |
| [16] | (g) Chen, J.; Tang, B.; Liu, X.; Lv, G.; Shi, Y.; Huang, T.; Xing, H.; Guo, X.; Hai, L.; Wu, Y. Org. Chem. Front. 2020, 7,2944. |
| [17] | (a) Vanos, C.; Lambert, T. Angew. Chem., Int. Ed. 2011, 50,12222. |
| [17] | (b) Wei, Y.; Zhao, W.-T.; Yang, Y.-L.; Zhang, Z.; Shi, M. ChemCatChem 2015, 7,3340. |
| [17] | (c) Shih, H.-W.; Prescher, J.A. J. Am. Chem. Soc. 2015, 137,10036. |
| [17] | (d) Aly, A.A.; Ramadan, M.; Al-Aziz, M.A.; Fathy, H.M.; Br?se, S.; Brown, A.B.; Nieger, M. J. Chem. Res. 2016, 40,637. |
| [17] | (e) Cunha, S.; Serafim, J.C.; de Santana, L.L. B.; Damasceno, F.; Correia, J.T. M.; Santos, A.O.; Oliveira, M.; Ribeiro, J.; Amparo, J.; Costa, S.L. J. Heterocycl. Chem. 2017, 54,3700. |
| [17] | (f) El-Sheref, E.M. J. Sulfur Chem. 2017, 38,625. |
| [17] | (g) Li, X.; Han, C.; Yao, H.; Lin, A. Org. Lett. 2017, 19,778. |
| [17] | (h) Xu, J.; Cao, J.; Fang, C.; Lu, T.; Du, D. Org. Chem. Front. 2017, 4,560. |
| [17] | (i) Reitel, K.; Kriis, K.; J?rving, I.; Kanger, T. Chem. Heterocycl. Compd. 2018, 54,929. |
| [17] | (j) Matsuda, T.; Tabata, Y.; Suzuki, H. New J. Chem. 2018, 42,19178. |
| [17] | (k) Wu, J.; Gao, W.-X.; Huang, X.-B.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Org. Lett. 2020, 22,5555. |
| [17] | (l) Liu, S.-W.; Yuan, C.; Jiang, X.-F.; Wang, X.-X.; Cui, H.-L. Asian J. Org. Chem. 2020, 9,82. |
| [17] | (m) Yang, Y.-F.; Huang, X.-B.; Gao, W.-X.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Org. Biomol. Chem. 2020, 18,5822. |
| [17] | (n) Jamshaid, F.; Kondakal, V.V. R.; Newman, C.D.; Dobson, R.; Jo?o, H.; Rice, C.R.; Mwansa, J.M.; Thapa, B.; Hemming, K. Tetrahedron 2020,131570. |
| [18] | (a) Zhu, B.Z.; Shao, B.; Li, F.; Liu, Y.X.; Huang, C.H. Acta Chim. Sinica 2015, 73,765. (in Chinese) |
| [18] | ( 朱本占, 邵波, 李锋, 刘玉祥, 黄春华, 化学学报, 2015, 73,765.) |
| [18] | (b) Gu, Y.Y.; Lü, X.Q.; Ma, X.D.; Zhang, H.J.; Ji, Y.Y.; Ding, W.J.; Shen, L. Chin. J. Org. Chem. 2020, 40,95. (in Chinese) |
| [18] | ( 顾依钰, 吕晓庆, 马晓东, 张浩健, 嵇媛媛, 丁婉婧, 沈立, 有机化学, 2020, 40,95.) |
| [19] | Peart, P.A.; Tovar, J.D. J. Org. Chem. 2010, 75,5689. |
| [20] | Jeffrey, C.S.; Barnes, K.L.; Eickhoff, J.A.; Carson, C.R. J. Am. Chem. Soc. 2011, 133,7688. |
| [21] | (a) Foucaud, A.; Bakouetila, M. Synthesis 1987,854. |
| [21] | (b) Li, C.; Jiang, K.; Ouyang, Q.; Liu, T.-Y.; Chen, Y.-C. Org. Lett. 2016, 18,2738. |
/
| 〈 |
|
〉 |