

深海真菌Diaporthe phaseolorum FS459的聚酮类化合物研究
收稿日期: 2020-10-31
修回日期: 2020-12-14
网络出版日期: 2020-12-31
基金资助
国家自然科学基金(41906106); 国家自然科学基金(31272087); 广东省海洋经济发展专项基金(GDNRC[2020]042); 广东省科学院建设国内一流研究机构行动专项(2019GDASYL-0103007); 广东省自然科学基金(2016A030312014)
Polyketides from the Deep-Sea-Derived FungusDiaporthe phaseolorum FS459
Received date: 2020-10-31
Revised date: 2020-12-14
Online published: 2020-12-31
Supported by
National Natural Science Foundation of China(41906106); National Natural Science Foundation of China(31272087); Guangdong Provincial Special Fund for Marine Economic Development Project(GDNRC[2020]042); Guangdong Academy of Sciences Project of Science and Technology Development(2019GDASYL-0103007); Natural Science Foundation of Guangdong Province(2016A030312014)
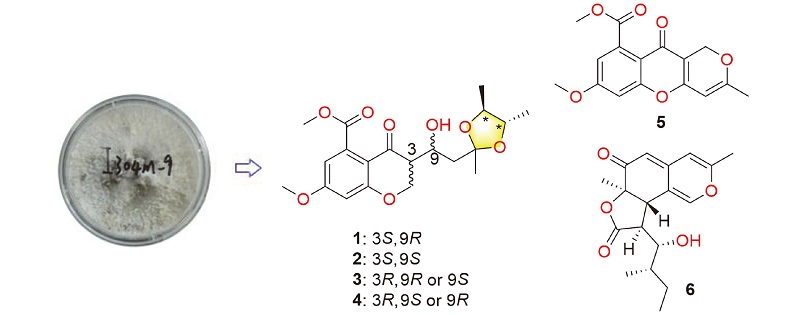
从深海真菌Diaporthe phaseolorum FS459中分离出10个聚酮化合物,包括3个新的色酮衍生物(1、2和5),一对新的差向异构体混合物(3和4), 1个新的Azaphilone类似物(6)及4个已知的化合物(7~10). 它们的结构是基于综合波谱分析进行确定, 同时其绝对构型通过对比实测电子圆二色谱(ECD)曲线与量子化学计算得出的理论值来确定. 化合物1~4具有罕见的2?,3?-二甲基二氧戊环结构单元. 此外, 本研究还评估了化合物1~10的体外细胞毒性、抗菌活性及NO产生的抑制效果.
关键词: 聚酮; 深海真菌; Diaporthe phaseolorum; 细胞毒
胡彩云 , 李赛妮 , 陈玉婵 , 高晓霞 , 刘昭明 , 章卫民 . 深海真菌Diaporthe phaseolorum FS459的聚酮类化合物研究[J]. 有机化学, 2021 , 41(4) : 1591 -1598 . DOI: 10.6023/cjoc202010046
Ten polyketides, including three new chromone derivatives (1, 2 and 5), a pair of new epimer mixtures (3 and 4), a new azaphilone analogue (6) and four known compounds (7~10) were isolated from the deep-sea-derived fungus Diaporthe phaseolorumFS459. Their structures were elucidated by extensive spectroscopic analysis, while the absolute configurations were established by comparison of experimental and quantum chemical calculated electronic circular dichroism (ECD) spectra. Compounds 1~4 possessed a rare 2?,3?-dimethyl-dioxolane moiety. Thein vitro cytotoxicity, antibacterial activities and NO production inhibitory effects of compounds 1~10 were evaluated.

Key words: polyketides; deep-sea-derived fungus; Diaporthe phaseolorum; cytotoxicity
| [1] | Hussain, H.; Al-Sadi, A.; Schulz, B.; Steinert, M.; Khan, A.; Green, I.; Ahmed, I. Future Med. Chem. 2019, 9,1631. |
| [2] | Walsh, C. Nat. Rev. Microbiol. 2003, 1,65. |
| [3] | Keller, N.P.; Turner, G.; Bennett, J.W. Nat. Rev. Microbiol. 2005, 3,937. |
| [4] | Weissman, K.J.; Leadlay, P. F.; Nat. Rev. Microbiol. 2005, 3,925. |
| [5] | Crawford, J.M.; Townsend, C.A. Nat. Rev. Microbiol. 2010, 8,879. |
| [6] | Mahé, S.; Rédou, V.; Calvez, T.L.; Vandenkoornhuyse, P.; Burgaud, G. Fungi in Deep-Sea Environments and Metagenomics, Vol. 12, Ed.: Martin, F., John Wiley & Sons, Inc., 2013, p. 325. |
| [7] | Wang, Y.-T.; Xue, Y.-R.; Liu, C.-H. Mar. Drugs 2015, 21,21. |
| [8] | Li, Q.; Xu, W.; Fan, R.; Zhang, J.; Li, Y.; Wang, X.; Han, S.; Lin, W.; Pan, M.; Cheng, Z. J. Nat. Prod. 2020, 83,2679. |
| [9] | Jiao, W.-H.; Xu, Q.J.; Ge, G.B.; Shang, R.Y.; Zhu, H.R.; Liu, H.Y.; Cui, J. Org. Lett. 2020, 22, 1825. |
| [10] | Wang, F.; Sarotti, A.M.; Jiang, G.D.; Huguet-Tapia, J.C.; Zheng, X.H.; Li, C.S.; Ding, Y.S.; Cao, S.G. Org. Lett. 2020, 22,4408. |
| [11] | Xu, J.; Tan, H.; Chen, Y.; Li, S.; Huang, Z.; Guo, H.; Li, H.; Gao, X.; Liu, H.; Zhang, W. Org. Chem. Front. 2018, 5,1792. |
| [12] | Chen, S.; Liu, Z.; Tan, H.; Chen, Y.; Li, S.; Li, H.; Zhuang, S.; Liu, H.; Zhang, W. Org. Chem. Front. 2020, 7,557. |
| [13] | Cui, H.; Ding, M.; Huang, D.; Zhang, Z.; Liu, H.; Huang, H.; She, Z. RSC Adv. 2017, 7,20128. |
| [14] | Slade, D.; Ferriera, D.; Marais, J.P. J. Phytochemistry 2005, 66,2117. |
| [15] | L?sgen, S.; Schl?rke, O.; Meindl, K.; Herbst-Irmer, R.; Zeeck, A.; Eur. J. Org. Chem. 2007, 13,2191. |
| [16] | Huang, X.; Feng, X.; Xiao, Z.; Liu, L.; Li, H.; Ma, L.; Lu, Y.; Ju, J.; She, Z.; Lin, Y. J. Prod. Nat. 2011, 74,997. |
| [17] | Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J. A., Jr.; Vreven, T. et al. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, USA, 2013. |
| [18] | Bruhn, T.; Schauml?ffel, A.; Hemberger, Y.; Bringmann, G. Chirality 2013, 25,243. |
| [19] | Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. J. Natl. Cancer Inst. 1990, 82,1107. |
| [20] | Liu, Z.; Qiu, P; Liu, H.; Li, J.; Shao, C.; Yan, T.; Cao, W.; She, Z. Chem 2019, 88,102973. |
| [21] | Tran, T.D.; Olsson, M.A.; Choudhury, M.A.; McMillan, D.J.; Cullen, J.K.; Parsons, P.G.; Bernhardt, P.V.; Reddell, P.W.; Ogbourne, S.M. J. Nat. Prod. 2019, 82,2809. |
/
| 〈 |
|
〉 |