

三氟乙基酮亚胺参与的催化不对称反应研究进展
收稿日期: 2020-11-04
修回日期: 2020-12-10
网络出版日期: 2020-12-31
基金资助
国家自然科学基金(21907044); 云南省基础研究计划项目(2019FB124); 云南省科技厅-昆明医科大学应用基础研究联合专项基金项目(2017FE468(-138)); 云南省教育厅科学研究基金(2016ZZX089); 西南林业大学西南地区林业生物质高效利用国家林和草原局重点实验室开放基金(2019-KF18); 西南林业大学西南地区林业生物质高效利用国家林和草原局重点实验室开放基金(2020-KF06)
Recent Advances in Catalytic Asymmetric Reactions Involving Trifluoroethyl Ketimines
Received date: 2020-11-04
Revised date: 2020-12-10
Online published: 2020-12-31
Supported by
National Natural Science Foundation of China(21907044); Yunnan Fundamental Research Projects(2019FB124); Yunnan Provincial Science and Technology Department-Kunming Medical University Applied Basic Research Joint Special Fund Project(2017FE468(-138)); Yunnan Provincial Department of Education Science Research Fund Project(2016ZZX089); Open Fund of Key Laboratory of State Forestry and Grassland Adminstration on Highly-Efficient Utilization of Forestry Biomass Resources in Southwest China, Southwest Forestry University(2019-KF18); Open Fund of Key Laboratory of State Forestry and Grassland Adminstration on Highly-Efficient Utilization of Forestry Biomass Resources in Southwest China, Southwest Forestry University(2020-KF06)
孙忠文 , 张聪聪 , 陈丽君 , 谢惠定 , 柳波 , 刘丹丹 . 三氟乙基酮亚胺参与的催化不对称反应研究进展[J]. 有机化学, 2021 , 41(5) : 1789 -1803 . DOI: 10.6023/cjoc202011005
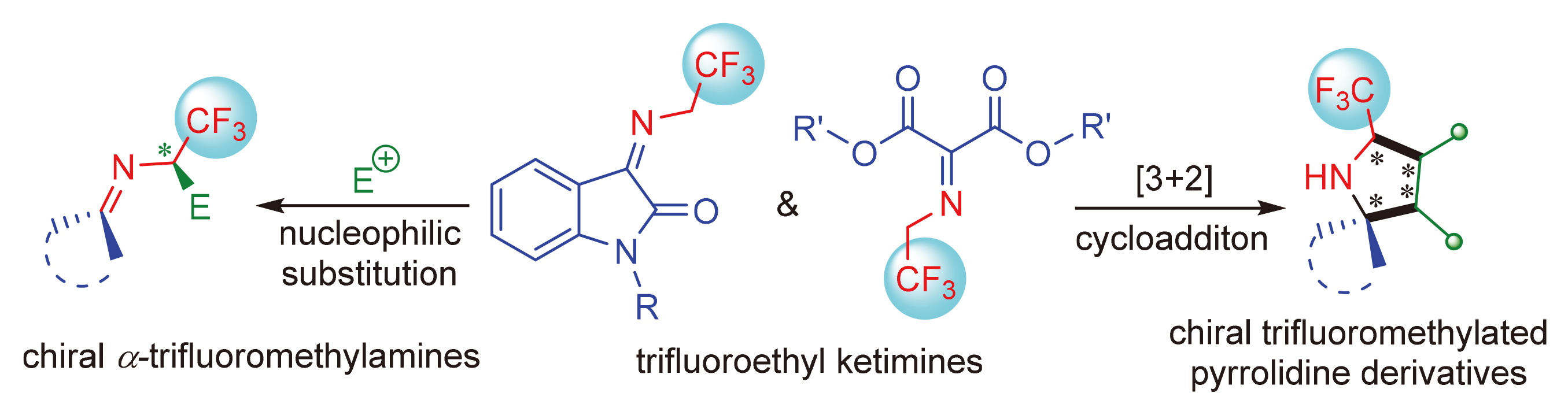
The fluorine atoms or fluorine-containing groups are widely found in biologically molecules in the fields of materials science, pharmaceutical chemistry, etc. The properties of trifluoroethylketimines provide both electrophilic and nucleophilic centers, and become an excellent 1,3-dipole, which possessed high research value in catalytic asymmetric reactions of construction of trifluoromethyl stereocenters. Based on the substrates and reaction types of trifluoroethylketimine, the research progress of catalytic asymmetric reactions involving trifluoroethylketimine in recent five years is reviewed, and the future development of this field is prospected.
| [1] | (a) Furuya, T.; Kamlet, A. S.; Ritter, T. Natrue 2011, 473, 470. |
| [1] | (b) Merino, F.; Nevado, C. Chem. Soc. Rev. 2014, 43, 6598. |
| [1] | (c) Usachev, B. I. J. Fluorine Chem. 2015, 175, 36. |
| [1] | (d) Huang, S.-C.; Schlinquer, C.; Poisson, T.; Pannecoucke, X.; Charette, A. B.; Jubault, P. Chem.-Eur. J. 2018, 24, 10339. |
| [1] | (d) He, X.-H.; Ji, Y.-L.; Peng, C.; Han, B. Adv. Synth. Catal. 2019, 361, 1923. |
| [2] | (a) Corbett, J. W.; Ko, S. S.; Rodgers, J. D.; Gearhart, L. A.; Magnus, N. A.; Bacheler, L. T.; Diamond, S.; Jeffrey, S.; Klabe, R. M.; Cordova, B. C.; Garber, S.; Logue, K.; Trainor, G. L.; Anderson, P. S.; Erickson-Viitanen, S. K. J. Med. Chem. 2000, 43, 2019. |
| [2] | (b) Jlalia, I.; Lensen, N.; Chaume, G.; Dzhambazova, E.; Astasidi, L.; Hadjiolova, R.; Bocheva, A.; Brigaud, T. Eur. J. Med. Chem. 2013, 62, 122. |
| [2] | (c) Liu, Y.; Chen, J.-L.; Wang, G.-H.; Sun, P.; Huang, H.; Qing, F.-L. Tetrahedron Lett. 2013, 54, 5541. |
| [2] | (d) Guillaume, M.; Benoit, C.; Sebastien, C.; Philippe, G.; Pierre, B. J.; Daniele, B. D. J. Med. Chem. 2004, 47, 2694. |
| [2] | (e) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455. |
| [3] | (a) Timperley, C.M. Waters, M. J. Fluorine Chem. 2005, 126, 1144. |
| [3] | (b) Morandi, B.; Carreira, C. M. Angew. Chem., Int. Ed. 2010, 49, 119. |
| [3] | (c) Morandi, B.; Mariampillai, B.; Carreira, C. M. Angew. Chem., Int. Ed. 2011, 50, 1101. |
| [3] | (d) Li, F.; Nie, J.; Dun, L.; Zheng, Y.; Ma, J.-A. Angew. Chem., Int. Ed. 2013, 52, 6255. |
| [3] | (e) Molander, G. A.; Ryu, D. Angew. Chem., Int. Ed. 2014, 53, 14181. |
| [3] | (f) Brusoe, A.T; Hartwig, J. F. J. Am. Chem. Soc. 2015, 137, 8460. |
| [3] | (g) Li, S.; Cao, W.-J.; Ma, J.-A. Synlett 2017, 28, 673. |
| [3] | (h) Kotozaki, M.; Chanthamath, S.; Fujii, T.; Shibatomi, K.; Iwasa, S. Chem. Commun. 2018, 54, 5110. |
| [3] | (i) Zhang, X.-W.; Hu, W.-L.; Chen, S.; Hu, X.-G. Org. Lett. 2018, 20, 860. |
| [3] | (j) Gui, H.-Z.; Wei, Y.; Shi, M. Chem.-Asian J. 2020, 15, 1225. |
| [4] | Ma, M.-X.; Zhu, Y.-Y.; Sun, Q.-T.; Li, X.-Y.; Su, J.-H.; Zhao, L.; Zhap, Y.-Y.; Qiu, S.; Yan, W.-J.; Wang, K.-R.; Wang, R. Chem. Commun. 2015, 51, 8789. |
| [5] | Zhi, Y.; Zhao, K.; Liu, Q.; Wang, A.; Enders, D. Chem. Commun. 2016, 52, 14011. |
| [6] | Dong, Z.-H.; Zhu, Y.-Y.; Li, B.-Y.; Wang, C.; Yan, W.-J.; Wang, K.-R.; Wang, R. J. Org. Chem. 2017, 82, 3482. |
| [7] | Sun, Q.-T.; Li, X.-Y.; Su, J.-H.; Zhao, L.; Ma, M.-X.; Zhu, Y.-Y.; Zhao, Y.-Y.; Zhu, R.-R.; Yan, W.-J.; Wang, K.-R.; Wang, R. Adv. Synth. Catal. 2015, 357, 3187. |
| [8] | You, Y.; Lu, W.-Y.; Wang, Z.-H.; Chen, Y.-Z.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2018, 20, 4453. |
| [9] | Gao, X.-Y.; Yan, R.-J.; Xiao, B.-X.; Du, W.; Albrecht, L.; Chen, Y.-C. Org. Lett. 2019, 21, 9628. |
| [10] | Zhou, C.-C.; Han, Y.-Y.; Zeng, C.-K.; Zhang, T.-Y.; Ye, J.-X. Chin. Chem. Lett. 2020, 31, 377. |
| [11] | Choudhury, A.R; Mukherjee, S. Chem. Soc. Rev. 2020, 49, 6755. |
| [12] | Wang, Z.-H.; Wu, Z.-J.; Yue, D.-F.; Hu, W.-F.; Zhang, X.-M.; Xu, X.-Y.; Yuan, W.-C. Chem. Commun. 2016, 52, 11708. |
| [13] | (a) Song, Y.-X.; Du, D.-M. J. Org. Chem. 2018, 83, 9278. |
| [13] | (b) Lin, Y.; Song, Y.-X.; Du, D.-M. Adv. Synth. Catal. 2019, 361, 1064. |
| [13] | (c) An, T.-L.; Du, D.-M. ChemistrySelect 2019, 4, 11302. |
| [14] | (a) Li, B.-Y.; Gao, F.-Y.; Feng, X.; Sun, M.-M.; Guo, Y.-F.; Wen, D.-W.; Deng, Y.-B.; Huang, J.-Q.; Wang, K.-R.; Yan, W.-J. Org. Chem. Front. 2019, 6, 1567. |
| [14] | (b) Wang, C.; Wen, D.-W.; Chen, H.; Deng, Y.-B.; Liu, X.-T.; Liu, X.; Wang, L.; Gao, F.-Y.; Guo, Y.-F.; Sun, M.-M.; Wang, K.-R.; Yan, W.-J. Org. Biomol. Chem. 2019, 17, 5514. |
| [15] | Zhao, X.-Y.; Xiong, J.-L.; An, J.-K.; Yu, J.-C.; Zhu, L.-P.; Feng, X.; Jiang, X.-X. Org. Chem. Front. 2019, 6, 1989. |
| [16] | Liu, X.; Lu, D.-M.; Wu, J.-H.; Tan, J.-P.; Jiang, C.-H.; Gao, G.-W.; Wang, T.-L. Adv. Synth. Catal. 2020, 362, 1490. |
| [17] | (a) Sun, Q.-S.; Zhu, H.; Chen, Y.-J.; Yang, X.-D.; Sun, X.-W.; Lin, G.-Q. Angew. Chem., Int. Ed. 2015, 54, 13253. |
| [17] | (b) Zhu, L.-Y.; Chen, Q.-L.; Shen, D.; Zhang, W.-H.; Shen, C.; Zeng, X.-F.; Zhong, G.-F. Org. Lett. 2016, 18, 2387. |
| [17] | (c) Ren, J.-W.; Wang, J.; Xiao, J.-A.; Li, J.; Xiang, H.-Y.; Chen, X.-Q.; Yang, H. J. Org. Chem. 2017, 82, 6441. |
| [17] | (d) Yang, Q.-Q.; Xiao, W.; Du, W.; Qin, Q.-Y.; Chen, Y.-C. Chem. Commun. 2018, 54, 1129. |
| [17] | (e) Wang, C.-Y.; Wang, Z.-Y.; Yang, J.; Shi, S.-H.; Hui, X.-P. Org. Lett. 2020, 22, 4440. |
| [18] | (a) Huang, W.-J.; Chen, Q.; Lin, N.; Long, X.-W.; Pan, W.-G.; Xiong, Y.-S.; Weng, J.; Lu, G. Org. Chem. Front. 2017, 4, 472. |
| [18] | (b) Zhi, Y.; Zhao, K.; Essen, C. V.; Rissanen, K.; Enders, D. Synlett 2017, 28, 2876. |
| [18] | (c) Zhao, B.-L.; Du, D.-M. Adv. Synth. Catal. 2019, 361, 3412. |
| [18] | (d) Li, Yang, Hua, Y.-Z.; Lu, H.-J.; Liu, L.-T.; Wan, M.-C. Org. Lett. 2020, 22, 2527. |
| [19] | Zhu, W.-R.; Zhang, Z.-W.; Huang, W.-H.; Lin, N.; Chen, K.-B.; Wang, B.-C.; Weng, J.; Lu, G. Synthesis 2019, 51, 1969. |
| [20] | Li, X.-Y.; Sun, J.-H.; Liu, Z. -R.-J.; Zhu, Y.-Y.; Dong, Z.-H.; Qiu, S.; Wang, J.-Y.; Lin, L.; Shen, Z.-Q.; Yan, W.-J.; Wang, K.-R.; Wang, R. Org. Lett. 2016, 18, 956. |
| [21] | Shi, L.-M.; Sun, X.-S.; Chen, C.; Wang, Z.-F.; Tao, H.-Y.; Wang, C.-J. Org. Lett. 2019, 21, 4842. |
| [22] | Onywagusi, C. I.; Shao, X.-X.; Malcolmson, S. J. Org. Lett. 2020, 22, 681. |
| [23] | Zhu, W.-R.; Liu, K.; Weng, J.; Huang, W.-H.; Huang, W.-J.; Chen, Q.; Lin, N.; Lu, G. Org. Lett. 2020, 22, 5014. |
| [24] | (a) Zhu, Y.; Buchwald, S. L. J. Am. Chem. Soc. 2014, 136, 4500. |
| [24] | (b) Liu, J.; Cao, C.-G.; Sun, H.-B.; Zhang, X.; Niu, D.-W. J. Am. Chem. Soc. 2016, 138, 13103. |
| [25] | (a) Wang, Y.-W.; Deng, L.-F.; Zhang, X.; Niu, D.-W. Org. Lett. 2019, 21, 6951. |
| [25] | (b) Shen, C.; Wang, R.-Q.; Wei, L.; Wang, Z.-F.; Tao, H.-Y.; Wang, C.-J. Org. Lett. 2019, 21, 6940. |
| [26] | Wang, W.; Xiong, Q.; Gong, L.; Wang, Y.-W.; Liu, J.; Lan, Y.; Zhang, X. Org. Lett. 2020, 22, 5479. |
| [27] | Sun, J.-H.; Ma, Z.-L.; Li, X.-Y.; Li, L.; Shen, Z.-Q.; Yang, P.-J.; Li, Y.; Wang, H.-L.; Yan, W.-J.; Wang, K.-R.; Wang, R. Adv. Synth. Catal. 2016, 358, 3777. |
| [28] | Liu, Q.; Zhao, K.; Zhi, Y.; Raabe, G.; Enders, D. Org. Chem. Front. 2017, 4, 1416. |
| [29] | Li, B.-Y.; Liu, J.-K.; Gao, F. Y.; Sun, M.-M.; Guo, Y.-F.; Zhou, Y.; Wen, D.-W.; Deng, Y.-B.; Chen, H.; Wang, K.-R.; Yan, W.-J. Org. Biomol. Chem. 2019, 17, 2892. |
| [30] | Zhu, W.-Y.; Su, Q.; Lin, N.; Chen, Q.; Zhang, Z.-W.; Weng, J.; Lu, G. Org. Chem. Front. 2020, 7, 3452. |
/
| 〈 |
|
〉 |