

aza-Morita-Baylis-Hillman反应二次串联构建氨基衍生的1,6-二烯化合物
收稿日期: 2020-10-28
修回日期: 2020-12-30
网络出版日期: 2021-02-07
基金资助
国家自然科学基金(31702070)
Double aza-Morita-Baylis-Hillman Domino Reaction to Access Amino Derived 1,6-Dienes
Received date: 2020-10-28
Revised date: 2020-12-30
Online published: 2021-02-07
Supported by
National Natural Science Foundation of China(31702070)
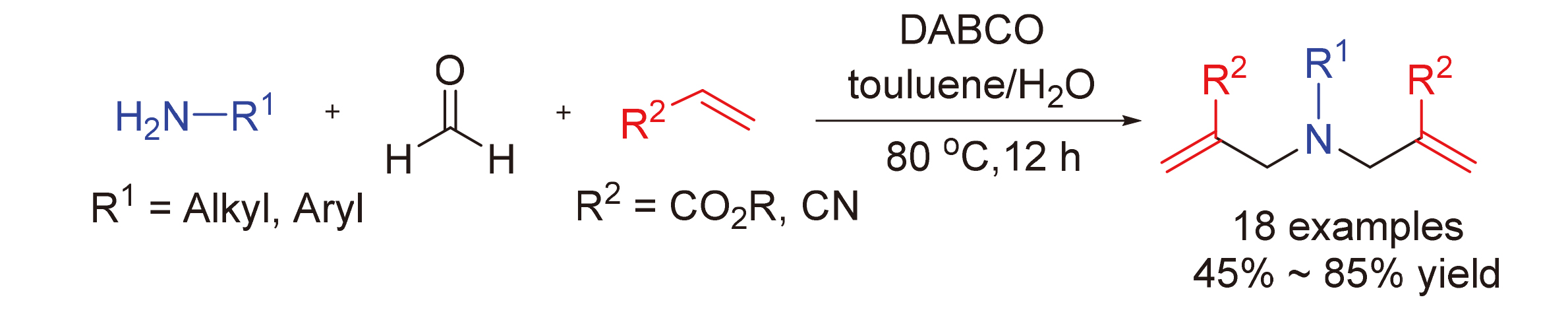
aza-Morita-Baylis-Hillman反应是一类非常重要的构建C—C键的人名反应, 被广泛应用于合成化学和药物化学领域. 报道了一类新颖的1,4-二氮杂二环[2.2.2]辛烷(DABCO)介导的二次aza-Morita-Baylis-Hillman串联反应. 该反应利用伯胺与甲醛能原位生成亚胺正离子的特征, 在甲苯与水的混合溶剂中, 实现了DABCO诱导的缺电子烯烃与亚胺正离子间的二次Mannich反应, 最终以中等到良好的产率获得了一系列氨基衍生的1,6-二烯化合物. 实验结果显示该三组分反应体系适用于一系列的苄胺、烷基胺和芳基胺底物, 有效避免了传统aza-Morita-Baylis-Hillman反应对底物胺的束缚, 为多样性1,6-二烯类化合物的合成提供了更加简洁的方法.
关键词: aza-Morita-Baylis-Hillman反应; 串联反应; 伯胺; 1,6-二烯
彭福涛 , 黄立梁 , 黄军海 , 冯煌迪 . aza-Morita-Baylis-Hillman反应二次串联构建氨基衍生的1,6-二烯化合物[J]. 有机化学, 2021 , 41(5) : 2001 -2007 . DOI: 10.6023/cjoc202010036
Aza-Morita-Baylis-Hillman reaction plays specific roles in the construction of C—C bond, and their applications in synthetic chemistry and pharmaceutical chemistry have also been well-documented. Herein, a novel 1,4-diazabicyclo- [2.2.2]octane (DABCO) mediated doubleaza-Morita-Baylis-Hillman cascade strategy toward synthetically important aza-MBH adducts is reported. Complementary to classical aza-MBH reaction, this protocol employs a wide variety of primary amines including benzyl, alkyl and aryl moieties as the reaction partners, giving an efficient alternative to produce amino derived 1,6-dienes in moderate to high yields in the presence of formaldehyde and α,β-unsaturated carbonyl compounds. Rather surprisingly, using an aqueous medium has proven to be successful in promoting the reaction efficiency and achieving a higher yield of target products. One-pot operation, high chemoselectivity, short reaction time, and broad substrate scope of primary amines exemplified the significant advances and practicability of this protocol.

| [1] | For selected reviews, see: (a) Basavaiah, D.; Jaganmohan Rao, A.; Satyanarayana, T. Chem. Rev. 2003, 103, 811. |
| [1] | (b) Declerck, V.; Martinez, J.; Lamaty, F. Chem. Rev. 2009, 109, 1. |
| [1] | (c) Xie, P. Z.; Huang, Y. Org. Biomol. Chem. 2015, 13, 8578. |
| [1] | (d) Wei, Y.; Shi, M. Chem. Rev. 2013, 113, 6659. |
| [1] | (e) Cui, P. M.; Wang, C.; Ma, J. J.; Zhang, Y. Q.; Gao, Y. J.; Chang, X. H.; Zhang, D. N.; Zhou, H.; Zhang, H. Y. Chin. J. Org. Chem. 2005, 25, 763. (in Chinese). |
| [1] | (崔朋雷, 王春, 马晶军, 张英群, 高勇军, 臧晓欢, 张冬暖, 周欣, 张红燕, 有机化学, 2005, 25, 763.) |
| [2] | (a) Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447. |
| [2] | (b) Zhou, L. J.; Yuan, C. H.; Zeng, Y.; Wang, Q. J.; Wang, C.; Liu, M.; Wang, W.; Wu, Y. J.; Zheng, B.; Guo, H. C. Org. Lett. 2019, 21, 4882. |
| [2] | (c) Peng, C.; Joy, A. Macromolecules 2014, 47, 125. |
| [2] | (d) Wu, M. Y.; Han, M. B.; Li, K. Z.; Wu, J.; Ding, K. L.; Lu, Y. X. J. Am. Chem. Soc. 2019, 141, 16362. |
| [3] | (a) Krafft, M. E.; Haxell, T. F. N. J. Am. Chem. Soc. 2005, 127, 10168. |
| [3] | (b) Li, Y. Q.; Wang, H. J.; Huang, Z. Z. J. Org. Chem. 2016, 81, 4429. |
| [3] | (c) Zhao, L. M.; Liu, K.; Li, D. F. J. Org. Chem. 2019, 84, 4429. |
| [3] | (d) Li, Y. Q.; Huang, Z. Z. Acta Chim. Sinica 2017, 75, 280. (in Chinese). |
| [3] | (李娅琼, 黄志真, 化学学报, 2017, 75, 280.) |
| [3] | (e) Mi, X. L.; Luo, S. Z.; Cheng, J. P. J. Org. Chem. 2005, 70, 2338. |
| [3] | (f) Yi, F. P.; Zhang, X.; Sun, H. Y.; Chen, S. H. Acta Chim. Sinica 2012, 70, 741. (in Chinese). |
| [3] | (易封萍, 张旋, 孙海洋, 陈世洪, 化学学报, 2012, 70, 741.) |
| [4] | (a) Bertenshaw, S.; Kahn, M. Tetrahedron Lett. 1989, 30, 2731. |
| [4] | (b) Ye, S. Q.; Wu, J. Tetrahedron Lett. 2009, 50, 6273. |
| [4] | (c) Zhang, X.; Zhou, Z.; Xu, H. Y.; Xu, X. F.; Yu, X. Y.; Yi, W. Org. Lett. 2019, 21, 7248. |
| [5] | (a) Chen, J.; Li, J. J.; Wang, J. Z.; Li, H.; Wang, W.; Guo, Y. W. Org. Lett. 2015, 17, 2214. |
| [5] | (b) Walton, M. C.; Yang, Y. F.; Hong, X.; Houk, K. N.; Overman, L. E. Org. Lett. 2015, 17, 6166. |
| [5] | (c) Jiang, B.; Xiao, B. X.; Ouyang, Q.; Liang, H. P.; Du, W.; Chen, Y. C. Org. Lett. 2019, 21, 3310. |
| [5] | (d) Patil, S. N.; Liu, F. J. Org. Chem. 2008, 73, 4476. |
| [5] | (e) Shi, F.; Luo, S. W.; Tao, Z. L.; He, L.; Yu, J.; Tu, S. J.; Gong, L. Z. Org. Lett. 2011, 13, 4680. |
| [5] | (f) Mergott, D. J.; Frank, S. A.; Roush, W. R. Org. Lett. 2002, 4, 3157. |
| [6] | Shi Shi. M.; Xu Y. M. J. Org. Chem. 2003, 68, 4784. |
| [7] | (a) Zhang, L.; Liu, H. L.; Qiao, G. Y.; Hou, Z. F.; Liu, Y.; Xiao, Y. M.; Guo, H. C. J. Am. Chem. Soc. 2015, 137, 4316. |
| [7] | (b) Qi, J. F.; Zheng, J.; Cui, S. L. Org. Lett. 2018, 20, 1355. |
| [8] | (a) Feng, H. D.; Jia, H. H.; Sun, Z. H. J. Org. Chem. 2014, 79, 11812. |
| [8] | (b) Feng, H. D.; Jia, H. H.; Sun, Z. H. Adv. Synth. Catal. 2015, 357, 2447. |
| [8] | (c) Zhang, Y. Z.; Huang, L. L.; Li, X. Y.; Wang, L.; Feng, H. D. J. Org. Chem. 2019, 84, 5046. |
| [8] | (d) Li, H. Q.; Feng, H. D.; Wang, F.; Huang, L. L. J. Org. Chem. 2019, 84, 10380. |
| [8] | (e) Feng, H. D.; Zhang, Y. Z.; Zhang, Z. D.; Chen, F. B.; Huang, L. L. Eur. J. Org. Chem. 2019,1931. |
| [8] | (f) Zhang, Y. Z.; Feng, H. D.; Liu, X. H.; Huang, L. L. Eur. J. Org. Chem. 2018,2039. |
| [8] | (g) Xu, X. J.; Van de Eycken, E. V.; Feng, H. D. Chin. J. Chem. 2020, 38, 1780. |
| [9] | (a) Xu, M. Y.; Jiang, W. T.; Li, Y.; Xu, Q. H.; Zhou, Q. L.; Yang, S.; Xiao, B. J. Am. Chem. Soc. 2019, 141, 7582. |
| [9] | (b) D?bbelin, M.; Azcune, I.; Bedu, M.; Ruiz de Luzuriaga, A.; Genua, A.; Jovanovski, V.; Caba?ero, G.; Odriozola, I. Chem. Mater. 2012, 24, 1583. |
| [9] | (c) Yan, T. B.; Liu, Y. H.; Shen, Y. H. Chin. J. Org. Chem. 2018, 38, 2491. (in Chinese). |
| [9] | (颜廷斌, 刘跃辉, 沈悦海, 有机化学, 2018, 38, 2491.) |
| [9] | (d) Huang, L.; Cai, Y.; Zhang, H.-J.; Zheng, C.; Dai, L.-X.; You, S.-L. CCS Chem. 2019, 1, 106. |
| [9] | (e) Zhu, M.; Zheng, C.; Zhang, X.; You, S.-L. J. Am. Chem. Soc. 2019, 141, 2636. |
| [10] | (a) Eren, T. N.; Graff, B.; Lalevee, J.; Avci, D. Prog. Org. Coat. 2019, 128, 156. |
| [10] | (b) Gao, S. F.; Zhang, Y. C.; Li, J. M.; Zhang, B. Yang, Y.; Hu, M. Q. Chin. J. Org. Chem. 2019, 39, 1953. (in Chinese). |
| [10] | (高粟繁, 张艳春, 李家明, 张斌, 杨雨, 胡孟奇, 有机化学, 2019, 39, 1953.) |
| [10] | (c) Zhao, Y. J.; Cao, Y. K.; Chend, H. Z.; Zhuang, F.; Wu, C.; Yoon, G.; Zhu, W. W.; Su, Y.; Zheng, S. Q.; Liu, Z. G.; Cheon, S. H. Bioorg. Med. Chem. 2019, 27, 963. |
| [11] | (a) Guo, Y. H.; Wang, G. D.; Wei, L.; Wan, J. P. J. Org. Chem. 2019, 84, 2984. |
| [11] | (b) Zheng, X. X.; Wan, J. P. Adv. Synth. Catal. 2019, 361, 5690. |
| [11] | (c) Fu, L. Q.; Cao, X. J.; Wan, J. P.; Liu, Y. Y. Chin. J. Chem. 2020, 38, 254. |
| [11] | (d) Chen, X. P.; Ma, Z. W.; Wang, C. C.; Liu, J. T.; Wu, J. S. Chin. J. Org. Chem. 2019, 39, 3176. (in Chinese). |
| [11] | (陈晓培, 马志伟, 王川川, 刘俊桃, 吴金松, 有机化学, 2019, 39, 3176.) |
| [12] | (a) Yu, C. Z.; Liu, B.; Hu, L. Q. J. Org. Chem. 2001, 66, 5413. |
| [12] | (b) Cai, J. X.; Zhou, Z. H.; Zhao, G. F.; Tang, C. C. Org. Lett. 2002, 4, 4723. |
| [12] | (c) Li, Y. X.; Liu, L.; Kong, D. L.; Wang, D.; Feng, W. C.; Yue, T.; Li, C. J. J. Org. Chem. 2015, 80, 6283. |
| [13] | (a) Wang, J. Y.; Shen, Q. Y.; Li, P. Z.; Peng, Y. Q.; Song, G. H. Org. Biomol. Chem. 2014, 12, 5597. |
| [13] | (b) Zhao, P. F.; Feng, H. D.; Pan, H. R.; Sun, Z. H.; Tong, M. C. Org. Chem. Front. 2017, 4, 37. |
| [13] | (c) Liu, B. Y.; Xu, X. J.; Huang, L. L.; Feng, H. D. Chin. J. Org. Chem. 2020, 40, 1290. (in Chinese). |
| [13] | (刘博瑜, 徐仙君, 黄立梁, 冯煌迪, 有机化学, 2020, 40, 1290.) |
| [14] | Pandey, A. K.; Han, S. H.; Mishra, N. K.; Kang, D.; Lee, S. H.; Chun, R.; Hong, S.; Park, J. S.; Kim, I. S. ACS Catal. 2018, 8, 742. |
| [15] | Shinohara, I.; Okue, M.; Yamada, Y.; Nagaoka, H. Tetrahedron Lett. 2003, 44, 4649. |
/
| 〈 |
|
〉 |