

硒化银新材料催化的醇氧化反应
收稿日期: 2020-11-08
修回日期: 2021-01-05
网络出版日期: 2021-02-22
Silver Selenide as the Novel Catalytic Material for Alcohol Oxidation
Received date: 2020-11-08
Revised date: 2021-01-05
Online published: 2021-02-22
刘峰 , 詹杰 , 孙扬阳 , 景崤壁 . 硒化银新材料催化的醇氧化反应[J]. 有机化学, 2021 , 41(5) : 2099 -2104 . DOI: 10.6023/cjoc202011012
By calcining selenium powder and silver powder at 150 ℃ under N2 protection, the novel material silver selenide (Ag/Se) could be easily fabricated. It possessed very strong oxygen carrier features and could activate the molecular oxygen to achieve the catalytic oxidation reactions. In this work, the Ag/Se-catalyzed alcohol oxidation reactions were investigated to afford a novel clean method for the synthesis of carbonyls. It could be applied for the oxidation reactions of primary and secondary alcohols, and could catalyze the selective oxidation of primary alcohols to synthesize aldehydes in moderate yields. Ag/Se as the novel Se-containing catalytic material may be comprehensively applied in future and significantly pushes forward the development of the chemistry of Se catalysis.
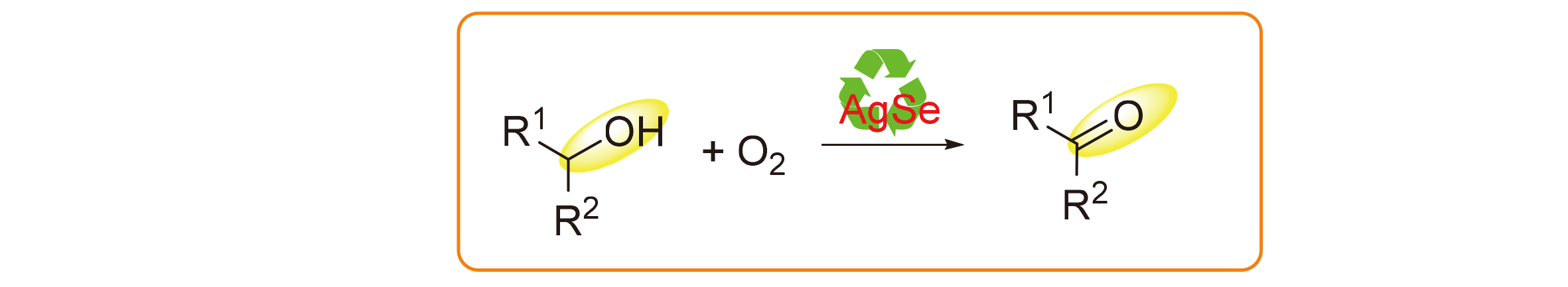
Key words: selenium; silver; catalyzed oxidation; alcohol; carbonyl
| [1] | (a) Cao, H.; Qian, R.; Yu, L. Catal. Sci. Technol. 2020, 10, 3113. |
| [1] | (b) Shao, L.; Li, Y.; Lu, J.; Jiang, X. Org. Chem. Front. 2019, 6, 2999. |
| [1] | (c) Singh, F. V.; Wirth, T. Catal. Sci. Technol. 2019, 9, 1073. |
| [1] | (d) Zheng, Y.; Wu, A.; Ke, Y.; Cao, H.; Yu, L. Chin. Chem. Lett. 2019, 30, 937. |
| [1] | (e) Yu, L. Chem. Reagents 2019, 41, 545. (in Chinese). |
| [1] | (俞磊, 化学试剂, 2019, 41, 545.) |
| [1] | (f) Cao, H.; Zhu, B.; Yang, Y.; Xu, L.; Yu, L.; Xu, Q. Chin. J. Catal. 2018, 39, 899. |
| [1] | (g) Guo, R.; Liao, L.; Zhao, X. Molecules 2017, 22, 835. |
| [2] | (a) Chen, C.; Cao, Y.; Wu, X.; Cai, Y.; Liu, J.; Xu, L.; Ding, K.; Yu, L. Chin. Chem. Lett. 2020, 31, 1078. |
| [2] | (b) Yu, L.; Cao, H.; Zhang, X.; Chen, Y.; Yu, L. Sustainable Energy Fuels 2020, 4, 730. |
| [2] | (c) Chen, X.; Mao, J.; Liu, C.; Chen, C.; Cao, H.; Yu, L. Chin. Chem. Lett. 2020, 31, 3205. |
| [2] | (d) Li, H.; Cao, H.; Chen, T.; Zhang, X.; Shi, Y. Mol. Catal. 2020, 483, 110715. |
| [3] | (a) Tiecco, M.; Testaferri, L.; Tingoli, M.; Bartoli, D. J. Org. Chem. 1990, 55, 4523. |
| [3] | (b) Ehara, H.; Noguchi, M.; Sayama, S.; Onami, T. J. Chem. Soc., erkin Trans. 1 2000,1429. |
| [4] | (a) Yu, L.; Wang, J.; Chen, T.; Ding, K.; Pan, Y. Chin. J. Org. Chem. 2013, 33, 1096. (in Chinese). |
| [4] | (俞磊, 王俊, 陈天, 丁克鸿, 潘毅, 有机化学, 2013, 33, 1096.) |
| [4] | (b) Yu, L.; Wu, Y.; Cao, H.; Zhang, X.; Shi, X.; Luan, J.; Chen, T.; Pan, Y.; Xu, Q. Green Chem. 2014, 16, 287. |
| [4] | (c) Zhang, X.; Ye, J.; Yu, L.; Shi, X.; Zhang, M.; Xu, Q.; Lautens, M. Adv. Synth. Catal. 2015, 357, 955. |
| [4] | (d) Yu, L.; Chen, F.; Ding, Y. ChemCatChem 2016, 8, 1033. |
| [4] | (e) Wang, F.; Xu, L.; Sun, C.; Xu, Q.; Huang, J.; Yu, L. Chin. J. Org. Chem. 2017, 37, 2115. (in Chinese). |
| [4] | (王芳, 徐林, 孙诚, 徐清, 黄杰军, 俞磊, 有机化学, 2017, 37, 2115.) |
| [4] | (f) Yang, Y.; Fan, X.; Cao, H.; Chu, S.; Zhang, X.; Xu, Q.; Yu, L. Catal. Sci. Technol. 2018, 8, 5017. |
| [4] | (g) Gao, G.; Han, J.; Yu, L.; Xu, Q. Synlett 2019, 30, 1703. |
| [4] | (h) Liu, C.; Mao, J.; Zhang, X.; Yu, L. Catal. Commun. 2020, 133, 105828. |
| [5] | Chen, C.; Cao, Z.; Zhang, X.; Li, Y.; Yu, L.; Jiang, X. Chin. J. Chem. 2020, 38, 1045. |
| [6] | (a) Deng, X.; Cao, H.; Chen, C.; Zhou, H.; Yu, L. Sci. Bull. 2019, 64, 1280. |
| [6] | (b) Deng, X.; Qian, R.; Zhou, H.; Yu, L. Chin. Chem. Lett. 2021, 32, 1029. |
| [6] | (c) Chen, Y.; Deng, X.; Jing, X.; Zhou, H. Chin. J. Org. Chem. 2020, 40, 4147. (in Chinese). |
| [6] | (陈颖, 邓鑫, 景崤壁, 周宏伟, 有机化学, 2020, 40, 4147.) |
| [7] | Cao, K.; Deng, X.; Chen, T.; Zhang, Q.; Yu, L. J. Mater. Chem. A 2019, 7, 10918. |
| [8] | (a) Yu, L.; Huang, X. Synlett 2007,1371. |
| [8] | (b) Usov, E. V.; Butov, A. A.; Chukhno, V. I.; Klimonov, I. A.; Kudashov, I. G.; Zhdanov, V. S.; Pribaturin, N. A.; Mosunova, N. A.; Strizhov, V. F. Atom. Energy 2018, 124, 287. |
| [8] | (c) Schweitzer-Chaput, B.; Sud, A.; Pintér, á.; Dehn, S.; Schulze, P.; Klussmann, M. Angew. Chem. Int. Ed. 2013, 52, 13228. |
| [8] | (d) Wei, Y.; Zou, Q.; Ye, P.; Wang, M.; Li, X.; Xu, A. Chemosphere 2018, 208, 358. |
| [9] | (a) Yu, L.; Ren, L.; Yi, R.; Guo, R. Synth. Commun. 2011, 41, 2530. |
| [9] | (b) Yu, L.; Huang, Y.; Bai, Z.; Zhu, B.; Ding, K.; Chen, T.; Ding, Y.; Wang, Y. J. Chin. Chem. Soc. 2015, 62, 479. |
| [10] | Zhang, X.; Sun, J.; Ding, Y.; Yu, L. Org. Lett. 2015, 17, 5840. |
| [11] | (a) Reich, H. J. Acc. Chem. Res. 1979, 12, 22. |
| [11] | (b) Liotta, D. Acc. Chem. Res. 1984, 17, 28. |
| [12] | (a) Chen, C.; Zhang, X.; Cao, H.; Wang, F.; Yu, L.; Xu, Q. Adv. Synth. Catal. 2019, 361, 603. |
| [12] | (b) Pinkus, A. G.; Ma, F. S. Y.; Meng, L. Y. C.; Chang, T. C. Org. Prep. Proced. Int. 2004, 36, 192. |
| [12] | (c) Spectral Database for Organic Compounds SDBS: http://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi. |
| [12] | (d) Wang, T.; Jing, X.; Chen, C.; Yu, L. J. Org. Chem. 2017, 82, 9342. |
| [12] | (e) Alizadeh, A.; Khodaei, M. M.; Eshghi, A. J. Org. Chem. 2010, 75, 8295. |
| [12] | (f) Qian, W.; Jin, E.; Bao, W.; Zhang, Y. Tetrahedron 2006, 62, 556. |
/
| 〈 |
|
〉 |