

齐墩果酸C3-糖缀合物的设计、合成及抗流感病毒活性研究
收稿日期: 2020-10-21
修回日期: 2020-12-15
网络出版日期: 2021-02-22
基金资助
云南省科技厅科技计划(2019FB125); 天然药物及仿生药物国家重点实验室开放基金(K202003)
Design, Synthesis and Anti-influenza A Virus Evaluation of Oleanolic Acid C3-Glycoconjugates
Received date: 2020-10-21
Revised date: 2020-12-15
Online published: 2021-02-22
Supported by
Science and Technology Plan Project of Yunnan Provincial Department of Science and Technology(2019FB125); Open-Fund Program of the State Key Laboratory of Natural and Biomimetic Drugs(K202003)
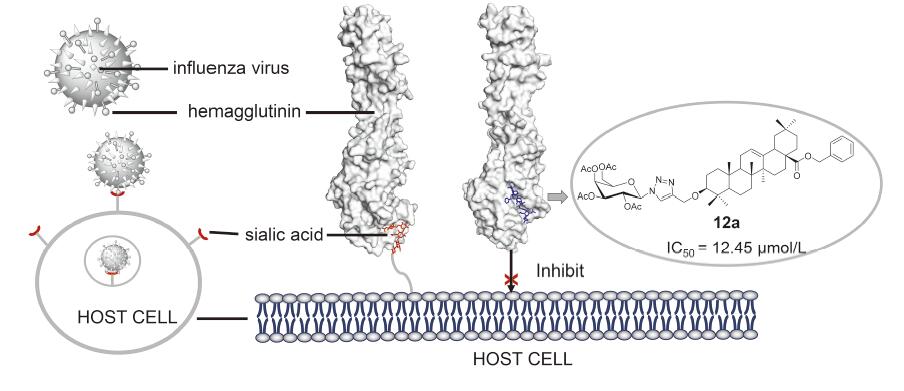
靶向流感病毒进入阶段的抑制剂是抗流感药物研发的热点. 前期研究发现, 齐墩果酸(OA)的C28位糖基化衍生物具有较强的抗流感病毒活性. 本研究采用Cu(I)催化叠氮-炔基Husigen环加成(CuAAC)反应设计合成了一系列齐墩果酸(C3)-糖缀合物7a~14c. 体外抗病毒研究发现, 齐墩果烷-12-烯-28-苄氧羰基-3-O-(4-亚甲基-1,2,3-三唑-1-(2,3,4,6-四-O-乙酰基-β-D-半乳糖苷)) (12a)具有显著的抗流感病毒活性, IC50为12.45 µmol•L –1, 且无明显的细胞毒性(CC50>100 µmol•L–1). 血凝抑制和分子对接实验表明, 化合物12a可能靶向流感病毒的膜蛋白血凝素(HA), 通过阻断HA与宿主细胞表面的唾液酸受体结合, 达到抑制流感病毒感染的目的. 本研究进一步完善了齐墩果酸及其衍生物抗流感病毒的构效关系, 为这类天然产物抗病毒的深入研究提供依据.
邵亮 , 杨帆 , 李唯嘉 , 俞飞 . 齐墩果酸C3-糖缀合物的设计、合成及抗流感病毒活性研究[J]. 有机化学, 2021 , 41(6) : 2454 -2466 . DOI: 10.6023/cjoc202010029
Inhibitors targeting the entry stage of influenza viruses are a hot spot in the development of anti-influenza drugs. Our previous studies showed that oleanolic acid (OA) C28 glycoconjugates displayed strong anti-influenza virus activity. In this paper, a series of oleanolic acid C3 glycoconjugates 7a~14c were designed and synthesized via copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) reaction. The anti-influenza activities of all these compounds were evaluatedin vitro. Among them, oleanane-12-enyl-28-benzyloxycarbonyl-3-O-(4-methylene-1,2,3-triazole-1-(2,3,4,6-tetra-O-acetyl-β-D-galactoside)) (12a) showed the strongest activity with an IC50 of 12.45 µmol•L –1, and no obvious cytotoxic effect on MDCK cells was observed at 100 µmol•L –1. Hemagglutination inhibition and molecular docking experiments indicated that compound 12a might target viral envelope hemagglutinin (HA), thus inhibiting the attachment of viruses to host cells. This study improved the structure-activity relationships of oleanolic acid and its derivatives against influenza virus, and provided a basis for further research on anti-virus by these natural products.

Key words: oleanolic acid; glycoconjugates; influenza virus; CuAAC reaction; inhibitors
| [1] | CDC. Influenza Type A Viruses[EB/OL]. [2017-04-19]. https: //www. cdc. gov/flu/avianflu/influenza-a-virus-subtypes. htm |
| [2] | Kilbourne; Edwin, D. Emerg Infect Dis. 2006, 12(1),9. |
| [3] | van Dongen, M.; Kadam, R. U.; Juraszek, J.; Lawson, E.; Brandenburg, B.; Schmitz, F.; Schepens, W.; Stoops, B.; van Diepen, H. A.; Jongeneelen, M.; Tang, C.; Vermond, J.; van Eijgen-Obregoso Real, A.; Blokland, S.; Garg, D.; Yu, W.; Goutier, W.; Lanckacker, E.; Klap, J. M.; Peeters, D.; Wilson, I. A. Science. 2019, 363(6431),6221. |
| [4] | Fang, Y.; Xiao, M.; Hu, A.; Ye, J.; Lian, W.; Liu, A. Chin J. Chem. 2016, 34,403. |
| [5] | Sheu, T. G.; Fry, A. M.; Garten, R. J.; Deyde, V. M.; Shwe, T.; Bullion, L.; Peebles, P. J.; Li, Y.; Klimov, A. I.; Gubareva, L. V. J Infect Dis. 2011, 203(1),13. |
| [6] | Hay, A. J.; Hayden, F. G. Lancet. 2013, 381(9885),2230. |
| [7] | Heo, Y. A. Drugs. 2018, 78(6),693. |
| [8] | Hayden, F. G.; & Shindo, N. Curr Opin Infect Dis. 2019, 32(2),176. |
| [9] | Vanderlinden, E.; Naesens, L. Med. Res. Rev. 2014, 34,301. |
| [10] | Zhao, X.; Li, R.; Zhou, Y.; Xiao, M.; Ma, C.; Yang, Z.; Zeng, S.; Du, Q.; Yang, C.; Jiang, H.; Hu, Y.; Wang, K.; Mok, C K P.; Sun, P.; Dong, J.; Cui, W.; Wang, J.; Tu, Y.; Yang, Z.; Hu, W. J Med Chem. 2018, 61(12),5187. |
| [11] | Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Yu, F.; Jiao, P.; Shi, Y.; Wang, H.; Xiao, S.; Fu, G.; Tian, K.; Wang, Y.; Guo, Z.; Ye, X.; Zhang, L.; Zhou, D. J. Med. Chem. 2014, 57,10058. |
| [12] | Li, S.; Jia, X.; Shen, X.; Wei, Z.; Jiang, Z.; Liao, Y.; Guo, Y.; Zheng, X.; Zhong, G.; Song, G. Bioorg Med Chem. 2017, 25(16),4384. |
| [13] | Song, G.; Shen, X.; Li, S.; Li, Y.; Si, H.; Fan, J.; Li, J.; Gao, E.; Liu, S. Eur J Med Chem. 2016, 119,109. |
| [14] | Song, G.; Shen, X.; Li, S.; Si, H.; Li, Y.; Luan, H.; Fan, J.; Liang, Q.; Liu, S. RSC Adv. 2015, 5,39145. |
| [15] | Su, Y.; Meng, L.; Sun, J.; Li, W.; Shao, L.; Chen, K.; Zhou, D.; Yang, F.; Yu, F. Eur J Med Chem. 2019, 182,111622. |
| [16] | Conte, G.; Cristiano, R.; Ely, F.; Gallardo, H. Synth. Commun. 2006, 36,951. |
| [17] | Chittaboina, S.; Xie, F., Wang, Q. (2005). Tetrahedron Letters 2005, 46(29),2331. |
| [18] | Maier, M. A.; Yannopoulos, C. G.; Mohamed, N.; Roland, A.; Fritz, H.; Mohan, V.; Just, G.; Manoharan, M. Bioconjug Chem. 2003, 14(1),18. |
| [19] | Zhao, S.; Zhang, S.; Xu, J.; Hu, L. Tetrahedron. 2020, 76(42),131517. |
| [20] | Tang, Y.; Zhang, S.; Chang, Y.; Fan, D.; Agostini, A.; Zhang, L.; Jiang, T. J Med Chem. 2018, 61(7),2937. |
| [21] | Andreeva, O.; Garifullin, B.; Sharipova, R.; Strobykina, I.; Sapunova, A.; Voloshina, A.; Belenok, M.; Dobrynin, A.; Khabibulina, L.; Kataev, V. J Nat Prod. 2020, 83(8),2367. |
| [22] | Tsoulougian, V.; Psykarakis, E.; Gimisis, T. Org Biomol Chem. 2019, 17(4),973. |
| [23] | Novoa, A.; Machida, T.; Barluenga, S.; Imberty, A.; Winssinger, N. Chembiochem. 2015, 15(14),2058. |
| [24] | Yu, F.; Zheng, Y.; Peng, Y.; Zhou, D.; Xiao, S. Design and synthesis of oleanolic acid rings a and c lactone derivatives. Chin. J. Org. Chem, 2016, 36,512(in Chinese). |
| [24] | (俞飞, 郑永祥, 彭逸云, 周德敏, 肖苏龙, 有机化学, 2016, 36,512.) |
| [25] | Li, W.; Yang, F.; Meng, L.; Sun, J.; Su, Y.; Shao, L.; Zhou, D.; Yu, F. Chem. Pharm Bull. 2019, 67(11),1201. |
/
| 〈 |
|
〉 |