

钯催化2-炔基芳基叠氮与丙烯酸衍生物反应合成1H-吲哚-3-丙烯酸酯
收稿日期: 2021-01-27
修回日期: 2021-04-10
网络出版日期: 2021-05-08
基金资助
国家自然科学基金(21302096); 江苏省自然科学基金(BK20171449); 国家一流学科资助项目
Palladium-Catalyzed Synthesis of 1H-Indol-3-yl Acrylates from 2-Alkynyl Arylazides and Acrylic Acids
Received date: 2021-01-27
Revised date: 2021-04-10
Online published: 2021-05-08
Supported by
National Natural Science Foundation of China(21302096); Natural Science Foundation of Jiangsu Province(BK20171449); National First-class Disciplines(PNFD)
李萍 , 周志强 , 杨帆 , 徐瑶 , 张小祥 . 钯催化2-炔基芳基叠氮与丙烯酸衍生物反应合成1H-吲哚-3-丙烯酸酯[J]. 有机化学, 2021 , 41(8) : 3235 -3241 . DOI: 10.6023/cjoc202101047
A novel method to prepare 1H-indol-3-yl acrylates from 2-alkynyl arylazides with acrylic acids catalyzed by Pd has been described. A variety of 1H-indol-3-yl acrylate derivatives were prepared under mild reaction conditions.
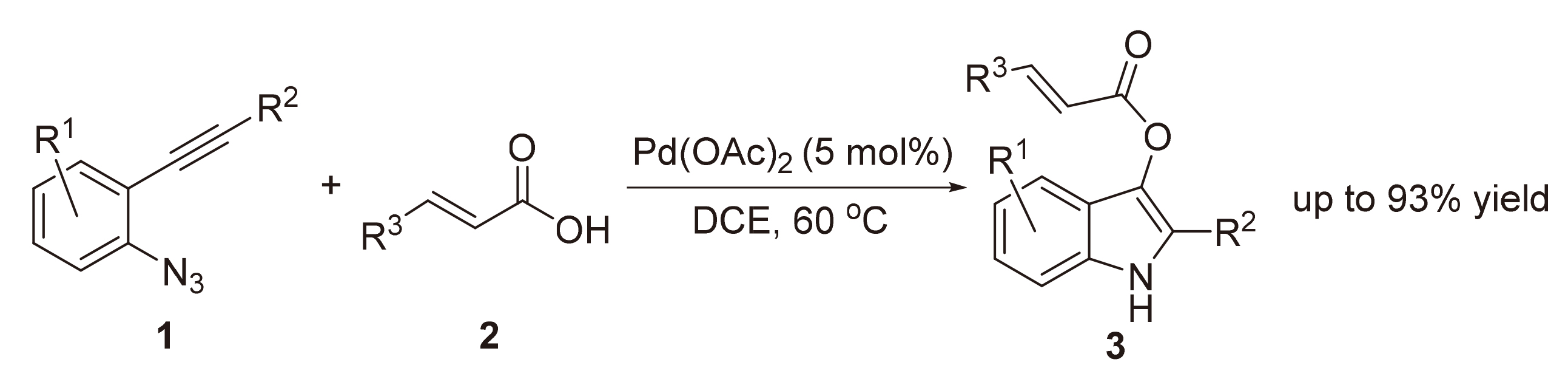
Key words: Pd-catalysis; α-imino palladium carbene; indole-3-acrylate; acrylic acid
| [1] | (a) Bandini, M.; Eichholzer, A. Angew. Chem., nt. Ed. 2009, 48, 9608. |
| [1] | (b) Joucla, L.; Djakovitch, L. Adv. Synth. Catal. 2009, 351, 673. |
| [1] | (c) Hong, J.; Xun, L.; Hong, W.; Huashan, Z.; Jieke, C. J. Wuhan Univ. (Nat. Sci. Ed.) 1998, 44, 175. |
| [1] | (d) Arnold, R. D.; Nutter, W. M.; Stepp, W. L. J. Org. Chem. 1959, 24, 117. |
| [1] | (e) Liu, K.; Wen, P.; Liu, J.; Huang, G. Synthesis 2010, 3623. |
| [1] | (f) Pohanka, M.; Drtinova, L. Talanta 2013, 106, 281. |
| [1] | (g) Li, X.; Yu, L.-M.; Wang, B.-J.; Xia, S.-W.; Zhao, H.-Z. Acta Chim. Sinica 2008, 66, 2507. (in Chinese) |
| [1] | (李霞, 于良民, 王宝娟, 夏树伟, 赵海洲, 化学学报, 2008, 66, 2507.) |
| [2] | (a) Sassano, M. F.; Schlesinger, A. P.; Jarstfer, M. B. Open Med. Chem. J. 2012, 6, 20. |
| [2] | (b) Masterova, N. S.; Ryabova, S. Y.; Alekseeva, L. M.; Evstratova, M. I.; Kiselev, S. S.; Granik, V. G. Russ. Chem. Bull. 2010, 59, 637. |
| [2] | (c) Dorjsuren, D.; Burnette, A.; Gray, G. N.; Chen, X.; Zhu, W.; Roberts, P. E.; Currens, M. J.; Shoemaker, R. H.; Ricciardi, R. P.; Sei, S. Antiviral Res. 2006, 69, 9. |
| [2] | (d) Perlmutter, P.; Puniani, E.; Westman, G. Tetrahedron Lett. 1996, 37, 1715. |
| [2] | (e) Krajnc, P.; Štefanec, D.; Brown, J. F.; Cameron, N. R. J. Polym. Sci., art A: Polym. Chem. 2005, 43, 296. |
| [2] | (f) Štefanec, D.; Krajnc, P. Polym. Inter. 2007, 56, 1313. |
| [2] | (g) Vasconcellos, M. L.; Silva, T. M.; Camara, C. A. Pest. Manage. Sci. 2006, 62, 288. |
| [2] | (h) Littke, A. F.; Fu, G. C. J. Org. Chem. 1999, 64, 10. |
| [3] | (a) Pohanka, M.; Drtinova, L. Talanta 2013, 106, 281. |
| [3] | (b) Nirogia, R. V. S.; Kambhampati, R.; Daulatabad, A. V.; Gudla, P.; Shaikh, M.; Achanta, P. K.; Shinde, A. K.; Dubey, P. K. J. Enzyme Inhib. Med. Chem. 2011, 26, 341. |
| [4] | Gherbovet, O.; Fauré, R.; Ferreira, F.; Durand, J.; Ragon, M.; Hostyn, G.; Record, E.; Bozonnet, S.; O’Donohue, M. J. J. Mol. Catal. B: Enzym. 2016, 126, 24. |
| [5] | Song, J.; Cui, J.; Liang, H.-B.; Liu, Q.; Dong, Y.-H.; Liu, H. Asian J. Org. Chem. 2018, 7, 341. |
| [6] | Zhu, J.-D.; Fang, S.-S.; Sun, K.-W.; Fang, C.; Lu, T.; Du, D. J. Org. Chem. 2018, 83, 10430. |
| [7] | (a) Li, P.; Zhu, B.; Xu, Y.; Zhou, Z.; Hu, G.; Yang, F.; Xu, S.; Zhang, X. Org. Chem. Front. 2020, 7, 3480. |
| [7] | (b) Li, P.; Yong, W.; Sheng, R.; Rao, W.; Zhu, X.; Zhang, X. Adv. Synth. Catal. 2019, 361, 201. |
| [8] | For recent selected examples of α-imino metal carbenes from 2-alkynyl arylazides: |
| [8] | (a) Lu, B.; Luo, Y.-D.; Liu, L.-Z.; Ye, L.-W.; Wang, Y.-Z.; Zhang, L.-M. Angew. Chem., nt. Ed. 2011, 50, 8358. |
| [8] | (b) Wetzel, A.; Gagosz, F. Angew. Chem., nt. Ed. 2011, 50, 7354. |
| [8] | (c) Hou, Z.; Oishi, S.; Suzuki, Y.; Kure, T.; Nakanishi, I.; Hirasawa, A.; Tsujimoto, G.; Ohno, H.; Fujii, N. Org. Biomol. Chem. 2013, 11, 3288. |
| [8] | (d) Tokimizu, Y.; Oishi, S.; Fujii, N.; Ohno, H. Org. Lett. 2014, 16, 3138. |
| [8] | (e) Zhou, Q.; Zhang, Z.; Zhou, Y.; Li, S.; Zhang, Y.; Wang, J. J. Org. Chem. 2017, 82, 48. |
| [8] | (f) Li, T.; Chen, B.-L.; Zhu, L.-L.; Chen, Z. Tetrahedron Lett. 2020, 61, 151851. |
| [8] | (g) Li, N.; Lian, X.-L.; Li, Y.-H.; Wang, T.-Y.; Han, Z.-Y.; Zhang, L.; Gong, L.-Z. Org. Lett. 2016, 18, 4178. |
| [8] | (h) Gronnier, C.; Boissonnat, G.; Gagosz, F. Org. Lett. 2013, 15, 4234. |
| [8] | (i) Shen, W.-B.; Sun, Q.; Li, L.; Liu, X.; Zhou, B.; Yan, J.-Z.; Lu, X.; Ye, L.-W. Nat. Commun. 2017, 8, 1748. |
| [8] | For recent selected examples of metal carbenes: |
| [8] | (j) Li, M.-L; Chen, M.-Q.; Xu, B.; Zhu, S.-F.; Zhou, Q.-L. Acta Chim. Sinica 2018, 76, 883. (in Chinese) |
| [8] | (李茂霖, 陈梦青, 徐彬, 朱守非, 周其林, 化学学报, 2018, 76, 883.) |
| [8] | (k) Qiu, D.; Qiu, M.-L.; Ma, R.; Zhang, Y.; Wang, J.-B. Acta Chim. Sinica 2016, 74, 472. (in Chinese) |
| [8] | (邱頔, 邱孟龙, 马戎, 张艳, 王剑波, 化学学报, 2016, 74, 472.) |
| [9] | Zhang, X.-X.; Sun, X.-P.; Zhang, H.-F.; Cui, X.-L.; Ma, M.-T. Chin. J. Org. Chem. 2015, 35, 1469. (in Chinese) |
| [9] | (张小祥, 孙小萍, 张海飞, 崔杏丽, 马猛涛, 有机化学, 2015, 35, 1469.) |
| [10] | Zhang, X.-X.; Li, P.; Lyu, C.; Yong, W.-X.; Li, J.; Zhu, X.-B.; Rao, W.-D. Org. Biomol. Chem. 2017, 15, 6080. |
| [11] | (a) Zhang, X.-X.; Sun, X.-P.; Fan, H.; Li, P.; Lyu, C.; Rao, W.-D. Eur. J. Org. Chem. 2016, 25, 426. |
| [11] | (b) Zhang, X.-X.; Sun, X.-P.; Fan, H.; Lyu, C.; Li, P.; Zhang, H.-F.; Rao, W.-D. RSC Adv. 2016, 6, 56319. |
| [12] | For recent selected examples on the generation of palladium carbenes: |
| [12] | (a) Ding, H.; Bai, S.; Lu, P.; Wang, Y. Org. Lett. 2017, 19, 4604. |
| [12] | (b) Shao, Z.; Zhang, H. Chem. Soc. Rev. 2012, 41, 560. |
| [12] | (c) Xiao, Q.; Zhang, Y.; Wang, J. Acc. Chem. Res. 2013, 46, 236. |
| [12] | (d) Xia, Y.; Zhang, Y.; Wang, J. ACS Catal. 2013, 3, 2586. |
| [12] | (e) Liu, Z.; Tan, H.; Fu, T.; Xia, Y.; Qiu, D.; Zhang, Y.; Wang, J. J. Am. Chem. Soc. 2015, 137, 12800. |
/
| 〈 |
|
〉 |