

铜催化2-羟基芳基烯胺酮经三氟甲基自由基加成串联环化反应合成3-三氟甲基色酮
Copper-Catalyzed the Synthesis of 3-Trifluoromethylchromone via Trifluoromethyl Radical Addition Tandem Cyclization Reaction of 2-Hydroxyphenyl Enaminones
Received date: 2021-02-01
Revised date: 2021-03-16
Online published: 2021-05-08
杜科莹 , 张展铭 , 盛卫坚 . 铜催化2-羟基芳基烯胺酮经三氟甲基自由基加成串联环化反应合成3-三氟甲基色酮[J]. 有机化学, 2021 , 41(8) : 3242 -3248 . DOI: 10.6023/cjoc202102007
Chromones have shown strong drug activity in anti-tumor, antibacterial and anti-inflammatory aspects for their unique skeleton structure. Using Cu(OAc)2 as catalyst, CF3SO2Na as trifluoromethyl radical source, tert-butanol peroxide (TBHP) as oxidant, 3-trifluoromethylchromone was synthesized by radical addition tandem cyclization reaction of 2-hydroxyphenyl enaminone under mild reaction conditions, and enaminone substrate has good functional tolerance.
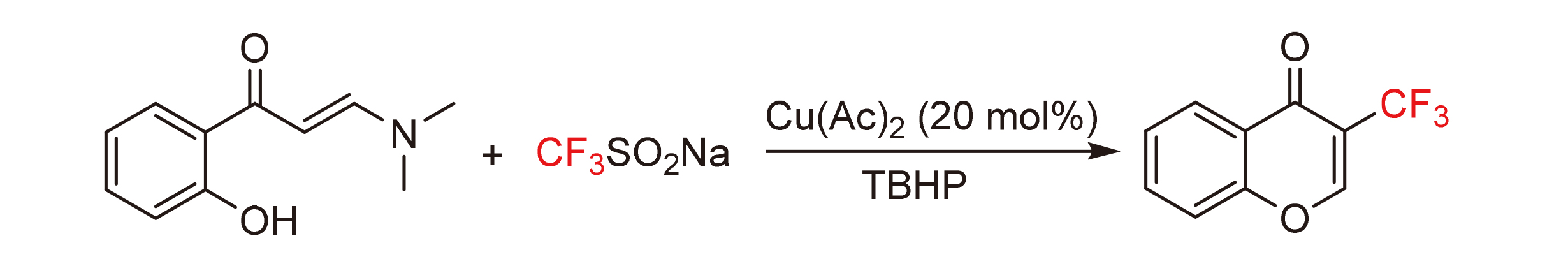
Key words: radical addition; trifluoromethyl; enaminone; chromones
| [1] | (a) Feng, L.; Maddox, M. M.; Alam, M. Z.; Tsutsumi, L. S.; Narula, G.; Bruhn, D. F.; Wu, X.; Sandhaus, S.; Lee, R. B.; Simmons, C. J.; Tse-Dinh, Y. C.; Hurdle, J. G.; Lee, R. E.; Sun, D. J. Med. Chem. 2014, 57, 8398. |
| [1] | (b) Matin, A.; Gavande, N.; Kim, M. S.; Yang, N. X.; Salam, N. K.; Hanrahan, J. R.; Roubin, R. H.; Hibbs, D. E. J. Med. Chem. 2009, 52, 6835. |
| [1] | (c) Zhou, T.; Shi, Q.; Lee, K. H. Tetrahedron Lett. 2010, 51, 4382. |
| [1] | (d) Keri, R. S.; Budagumpi, S.; Pai, R. K.; Balakrishna, R. G. Eur. J. Med. Chem. 2014, 78, 340. |
| [1] | (e) Lan, J. S.; Xie, S. S.; Huang, M.; Hu, Y. J.; Kong, L. Y.; Wang, X. B. MedChemComm 2015, 6, 1293. |
| [1] | (f) Gobbi, S.; Hu, Q.; Zimmer, C., Engel, M.; Belluti, F.; Rampa, A.; Hartmann, R. W.; Bisi, A. J. Med. Chem. 2016, 59, 2468. |
| [2] | (a) Moriarty, R. M.; Prakash, O. J. Heterocycl. Chem. 1985, 22, 583. |
| [2] | (b) Klier, L.; Bresser, T.; Nigst, T. A.; Karaghiosoff, K.; Knochel, P. J. Am. Chem. Soc. 2012, 134, 13584. |
| [2] | (c) Inna, V.; Shlomo R. J. Org. Chem. 2014, 79, 7261. |
| [2] | (d) Zhao, W. N.; Xie, P.; Bian, Z. G.; Zhou, A. H.; Ge, H. B.; Zhang, M.; Ding, Y. C.; Zheng, L. J. Org. Chem. 2015, 80, 9167. |
| [2] | (e) Wan, J. P.; Zhong, S.; Guo, Y.; Wei, L. Eur. J. Org. Chem. 2017, 4401. |
| [2] | (f) Araujo, D. R.; Lima, Y. R.; Barcellos, A. M.; Jacob, R. G.; Silva, M. S.; Perin, G. ARKIVOC 2020, 6, 276. |
| [3] | (a) Panja, S. K.; Maiti, S.; Bandyopadhyay, C. J. Chem. Res. 2010, 555. |
| [3] | (b) Akram, M. O.; Berabc, S.; Patil, N. T. Chem. Commun. 2016, 52, 12306. |
| [3] | (c) Joussota, J.; Schoenfeldera, A.; Larquetouxb, L.; Nicolasb, M.; Suffert, J.; Blond, G. Synthesis 2016, 48, 3364. |
| [3] | (d) Yokoe, I.; Maruyama, K.; Sugita, Y.; Harashida, T.; Shirataki, Y. Chem. Pharm. Bull. 1994, 42, 1697. |
| [3] | (e) Gudipati, R.; Kandula, V.; Raghavulu, K.; Basavaiah, K.; Yennam, S.; Behera, M. ChemistrySelect 2020, 5, 7093 |
| [4] | (a) Hu, B.; Zhou, P.; Rao, K.; Yang, J.; Li, L.; Yan, S.; Yu, F. Tetrahedron Lett. 2018, 59, 1438. |
| [4] | (b) He, Z. H.; Liu, W. P.; Li, Z. P. Chem. Asian J. 2011, 6, 1340. |
| [4] | (c) Jiang, H. F.; Huang, W.; Yu, Y.; Yi, S. J.; Li, J. W.; Wu, W. Q. Chem. Commun. 2017, 53, 7473 |
| [5] | (a) Zhang, X. Z.; Ge, D. L.; Chen, S. Y.; Yu, X. Q. RSC Adv. 2016, 6, 66320. |
| [5] | (b) Yang, Z.; Hu, L.; Cao, T.; An, L.; Li, L.; Yang, T.; Zhou, C. New J. Chem. 2019, 43, 16441. |
| [5] | (c) Xiang, H.; Zhao, Q.; Tang, Z.; Xiao, J.; Xia, P.; Wang, C.; Yang, C.; Chen, X.; Yang, H. Org. Lett. 2017, 19, 146. |
| [5] | (d) Gao, Y.; Liu, Y.; Wan, J. P. J. Org. Chem. 2019, 84, 2243. |
| [5] | (e) Gao, H.; Hu, B.; Dong, W. H.; Gao, X. S.; Jiang, L. L.; Xie, X. M.; Zhang, Z. G. ACS Omega 2017, 2, 3168 |
| [6] | (a) Schlosser, M. Angew Chem., Int. Ed. 2006, 45, 5432. |
| [6] | (b) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359. |
| [6] | (c) Meanwell, N. A. J. Med. Chem. 2011, 54, 2529. |
| [6] | (d) Cametti, M.; Crousse, B.; Metrangolo, P.; Milani, R.; Resnati, G. Chem. Soc. Rev. 2012, 41, 31. |
| [6] | (e) Wang, J.; Sanchez-Rosello, M.; Acena, J. L.; delPozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432. |
| [7] | (a) Kino, T.; Nagase, Y.; Ohtsuka, Y.; Yamamoto, K.; Uraguchi, D.; Tokuhisa, K.; Yamakawa, T. J. Fluorine Chem. 2010, 131, 98. |
| [7] | (b) Choi, S.; Kim, Y. J.; Kim, S. M.; Yang, J. W.; Kim, S. W.; Cho, E. J. Nat. Commun. 2014, 5, 4881. |
| [7] | (c) Feng, Z.; Min, Q. Q.; Zhao, H. Y.; Gu, J. W.; Zhang, X. G. Angew. Chem., Int. Ed. 2015, 54, 1270. |
| [7] | (d) Straathof, N. J. W.; Cramer, S. E.; Hessel, V.; Noel, T. Angew. Chem., Int. Ed. 2016, 55, 15549. |
| [7] | (e) Thomas, R.; Eugen, L.; Christian, K.; Oliver, R. ACS Catal. 2018, 8, 3950 |
| [8] | (a) Satoshi, M.; Oscar, G. L.; Engle, K. M.; Stefan, V.; Katherine, W.; Gerasimos, R.; Veronique, G. Chem.-Eur. J. 2012, 18, s8583. |
| [8] | (b) Gao, B.; Zhao, Y. C.; Ni, C. F.; Hu, J. B. Org. Lett. 2014, 16, 102. |
| [8] | (c) Li, Y. W.; Lu, Y.; Qiu, G. Y. S.; Ding, Q. P. Org. Lett. 2014, 16, 4240. |
| [8] | (d) Ma, J. J.; Yi, W. B.; Lu, G. P.; Cai, C. Adv. Synth. Catal. 2015, 357, 3447. |
| [8] | (e) Gou, B. Q.; Yang, C.; Zhang, L.; Xia, W. J. Acta Chim. Sinica 2017, 75, 66. (in Chinese) |
| [8] | (苟宝权, 杨超, 张磊, 夏吾炯, 化学学报, 2017, 75, 66.) |
| [8] | (f) Kautzky, J. A.; Wang, T.; Evans, R. W.; MacMillan, D. W. C. J. Am. Chem. Soc. 2018, 140, 6522. |
| [8] | (g) Wang, Q.; Gao, K. C.; Zou, J. P.; Zeng, R. S. Chin. J. Org. Chem. 2018, 38, 863. (in Chinese) |
| [8] | (王清, 高克成, 邹建平, 曾润生, 有机化学, 2018, 38, 863.) |
| [8] | (h) Ge, J. Y.; Ding, Q. P.; Wang, X. H.; Peng, Y. Y. J. Org. Chem. 2020, 85, 7658. |
| [9] | (a) Yasu, Y.; Koike, T.; Akita, M. Chem. Commun. 2013, 49, 2037. |
| [9] | (b) Ge, G. C.; Huang, X. J.; Ding, C. H.; Wan, S. L.; Dai, L. X.; Hou, X. L. Chem. Commun. 2014, 50, 3048. |
| [9] | (c) Asano, M.; Tomita, R.; Koike, T.; Akita, M. J. Fluorine Chem. 2015, 179, 83. |
| [9] | (d) Tomita, R.; Yasu, Y.; Koike, T.; Akita, M. Angew. Chem., Int. Ed. 2014, 53, 7144 |
| [10] | (a) Pandey, V. K.; Anbarasan, P. RSC Adv. 2016, 6, 18525. |
| [10] | (b) Ye, Y. D.; Lee, S. H.; Sanford, M. S. Org. Lett. 2011, 13, 5464. |
| [10] | (c) Hafner, A.; Brase, S. Adv. Synth. Catal. 2011, 353, 163044. |
| [10] | (d) Wu, Y. B.; Lu, G. P.; Yuan, T.; Xu, Z. B.; Wan, L.; Cai, C. Chem. Commun. 2016, 52, 13668 |
| [11] | (a) Shen, W. G.; Wu, Q. Y.; Gong, X. Y.; Aoa, G. Z.; Liu, F. Green Chem. 2019, 21, 2983. |
| [11] | (b) Cao, X. H.; Pan, X. Q.; Zhou, P. J.; Zou, J. P.; Asekun, O. T. Chem. Commun. 2014, 50, 3359. |
| [11] | (c) Wei, W., Wen, J. W.; Yang, D. S.; Liu, X. X.; Guo, M. Y.; Dong, R. M.; Wang, H. J. Org. Chem. 2014, 79, 4225. |
| [11] | (d) Zhang, K.; Xu, X. H.; Qing, F. L. J. Org. Chem. 2015, 80, 7658. |
| [11] | (e) Yang, B.; Xu, X. H.; Qing, F. L. Org. Lett. 2015, 17, 1906. |
| [11] | (f) Tang, L.; Yang, Z.; Chang, X. P.; Jiao, J. C.; Ma, X. T.; Rao, W. H.; Zhou, Q. J. Org. Lett. 2018, 20, 6520. |
| [11] | (g) Shi, X. L.; Li, X. W.; Ma, L. N.; Shi, D. Y. Catalysts 2019, 9, 278. |
| [11] | (h) Wang, N.; Gu, Q. S.; Cheng, Y. F.; Li, L.; Li, Z. L.; Guo, Z.; Liu, X. Y. Chin. J. Org. Chem. 2019, 39, 200. (in Chinese) |
| [11] | (王娜, 顾强帅, 程永峰, 李磊, 李忠良, 郭臻, 刘心元, 有机化学, 2019, 39, 200.) |
| [12] | Friden-Saxin, M.; Seifert, T.; Landergren, M. R.; Suuronen, T.; Lahtela-Kakkonen, M.; Jarho, E. M.; Luthman, K. J. Med. Chem. 2012, 55, 7104. |
| [13] | Yu, Q.; Liu, Y. Y.; Wan, J. P. Org. Chem. Front. 2020, 7, 2770. |
| [14] | Bichovski, P.; Haas, T. M.; Kratzert, D.; Streuff, J. Chem.-Eur. J. 2015, 21, 2339. |
/
| 〈 |
|
〉 |