

熊果酸的结构修饰与生物活性研究进展
收稿日期: 2021-02-19
修回日期: 2021-03-17
网络出版日期: 2021-05-14
基金资助
中国博士后科学基金面上(2020M682311); 河南中医药大学博士基金(00104311-2019-33); 河南省科技攻关(202102310514)
Advances in the Study of Structural Modification and Biological Activities of Ursolic Acid
Received date: 2021-02-19
Revised date: 2021-03-17
Online published: 2021-05-14
Supported by
National Natural Science Foundation of China Postdoctoral Science Foundation(2020M682311); Doctorʼs Fund of Henan University of Medicine(00104311-2019-33); Award for Science and Technology Tackling Key Problems Program of Henan Province(202102310514)
刘改枝 , 李金鑫 , 史礼君 , 刘萌芽 , 蔡邦荣 . 熊果酸的结构修饰与生物活性研究进展[J]. 有机化学, 2021 , 41(8) : 2974 -2989 . DOI: 10.6023/cjoc202102032
Ursolic acid (UA) belongs to a group of pentacyclic triterpenoids extracted from medicinal plants, fruits and vegetables. In recent years, the pharmacological action of ursolic acid has been widely studied. It has been reported that UA has a variety of pharmacological activities, including anti-cancer, anti-inflammation, anti-oxidation, anti-bacterial, anti-diabetes and so on. In spite of that UA shows the extensive therapeutic potential as a drug candidate, the poor water solubility and low bioavailability of UA limit its clinical application. To address these issue, large numbers of UA-based derivatives with enhanced activities and better physicochemical character have been developed by structural modification of its parent skeleton. In this paper, the research progress of the synthesized UA derivatives with therapeutic potential along with their biological activities is reviewed, which provides reference for further drug development using UA as a lead compound.
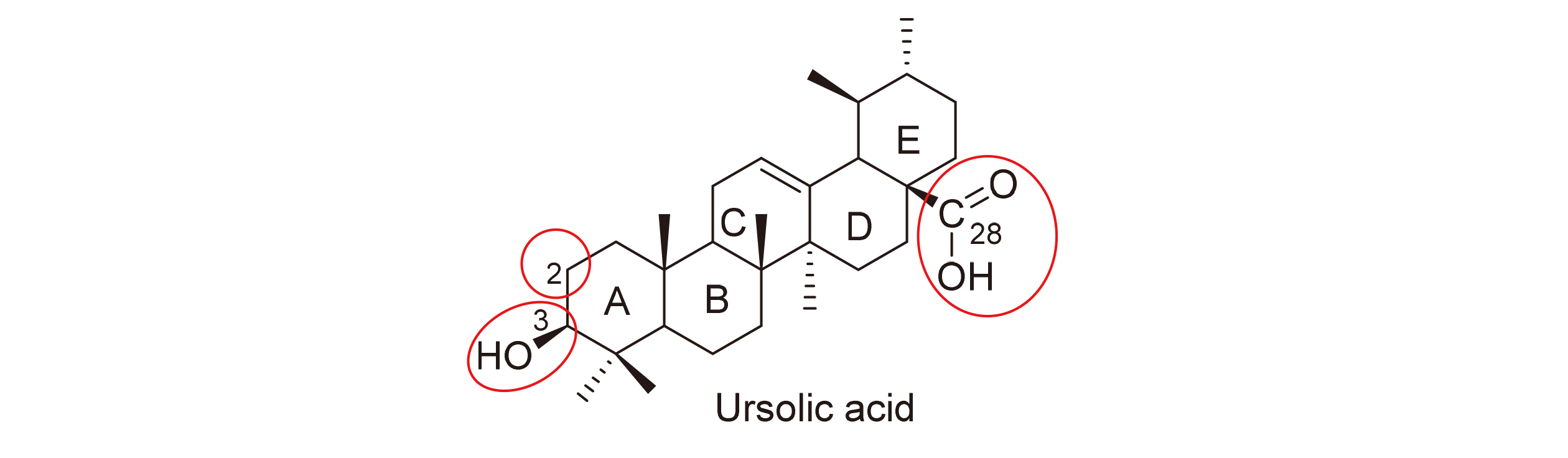
Key words: ursolic acid; structural modification; anti-tumor; anti-diabetic; anti-inflammatory; antiviral
| [1] | Tao, Y.-B.; Xing, Y.-L.; Fang, Z.-J.; Bi, L.-W.; Zhao, Z.-D. Chem. Ind. For. Prod. 2012, 32(1), 119. (in Chinese) |
| [1] | (陶渊博, 邢雅丽, 方芝娟, 毕良武, 赵振东, 林产化学与工业, 2012, 32(1), 119.) |
| [2] | Mlala, S.; Oyedeji, A. O.; Gondwe, M.; Oyedeji, O. O. Molecules 2019, 24(15), 2751. |
| [3] | López-Hortas, L.; P, P.-L.; Gonzá Lez-MuñOz, M. J.; Falqué, E.; DomíNguez, H. Food Res. Int. 2018, 103, 130. |
| [4] | Khwaza, V.; Oyedeji, O. O.; Aderibigbe, B. A. Int. J. Mol. Sci. 2020, 21(16), 5920. |
| [5] | Xiang, R.-Q.; Fan, Y. J. Pharm. Res. 2019, 38(2), 63. (in Chinese) |
| [5] | (向润清, 范源, 药学研究, 2019, 38(2), 63.) |
| [6] | Zhu, M. L.; Ge, W. Z.; Zhao, R. Acta Laser Biol. Sin. 2014, 23(5), 465. (in Chinese) |
| [6] | (朱美玲, 葛文中, 赵蕊, 激光生物学报, 2014, 23(5), 465.) |
| [7] | Wang, C.-Y.; Lin, C.-S.; Hua, C.-H.; Jou, Y.-J.; Liao, C.-R.; Chang, Y.-S.; Wan, L.; Huang, S.-H.; Hour, M.-J.; Wen, C.-L. Biomol. Ther. 2019, 27(1), 9. |
| [8] | Xu, J.; Wang, X.-D.; Zhang, H.-Y.; Yue, J.-Y.; Zhao, Y.-Q. Nat. Prod. Res. 2018, 34(6), 1. |
| [9] | Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Bioorg. Chem. 2019, 90, 103054. |
| [10] | Tu, H. Y.; Huang, A.; Wei, B. L.; Gan, K. H.; Hour, T. C.; Yang, S. C.; Pu, Y. S.; Lin, C. N. Bioorg. Med. Chem. 2009, 17(20), 7265. |
| [11] | Gou, W.-F.; Luo, N.; Wei, H.-Q.; Wu, H.-Y.; Li, Y.-L. Pharm. Biol. 2020, 58(1), 707. |
| [12] | Tian, T.; Liu, X.; Lee, E. S.; Sun, J.; Feng, Z.; Zhao, L.; Zhao, C. Arch. Pharmacal Res. 2017, 40(4), 1. |
| [13] | Li, X.; Bai, X.; Wu, K.; Wang, Y. J.; Shi, W. Q.; Li, Y.; Yin, S. F. Chin. J. Org. Chem. 2012, 32(4), 703. (in Chinese) |
| [13] | (李霞, 白雪, 吴科, 王裕军, 石万棋, 李颖, 尹述凡, 有机化学, 2012, 32(4), 703.) |
| [14] | Mallavadhani, U. V.; Mahapatra, A.; Pattnaik, B.; Vanga, N.; Suri, N.; Saxena, A. K. Med. Chem. Res. 2013, 22(3), 1263. |
| [15] | Liu, M. C.; Yang, S. J.; Jin, L. H.; Hu, D. Y.; Xue, W.; Song, B. A. Eur. J. Med. Chem. 2012, 58(12), 128. |
| [16] | Liu, M. C.; Yang, S. J.; Jin, L. H.; Hu, D. Y.; Xue, W.; Yang, S. Med. Chem. Res. 2016, 25(10), 2267. |
| [17] | Liu, H.; Li, G.-B.; Zhang, D.-L.; Liu, Y.-M.; Gao, N. J. Third Milit. Med. Univ. 2017, 39(19), 1960. (in Chinese) |
| [17] | (刘涵, 李国兵, 张定林, 刘毅敏, 高宁, 第三军医大学学报, 2017, 39(19), 1960.) |
| [18] | Chen, Y.-H.; Hou, X.-Y.; Zhi, D.-F.; Li, C.; Tian, T.; Sun, J.-Y.; Zhao, L.-X.; Zhao, C.-H. Chin. J. Org. Chem. 2016, 36(4), 795. (in Chinese) |
| [18] | (陈艳华, 侯熙彦, 支德福, 李常, 田甜, 孙竞阳, 赵龙铉, 赵春晖, 有机化学, 2016, 36(4), 795.) |
| [19] | Bai, K. K.; Chen, F. L.; Guo, H. H. Chin. J. New Drug 2012, 21(22), 2667. (in Chinese) |
| [19] | (白锴凯, 陈芬玲, 郭养浩, 中国新药杂志, 2012, 21(22), 2667.) |
| [20] | Cheng, W.; Dahmani, F. Z.; Zhang, J.; Xiong, H.; Wu, Y.; Yin, L.; Zhou, J.; Yao, J. Nanotechnol. 2017, 28(7), 075102. |
| [21] | Ahn, G. Y.; Paik, D. H.; Jeong, K. Y.; Baek, S. W.; Kang, R. H.; Lee, E. S.; Choi, S. W. Macromol. Res. 2018, 26(7), 1. |
| [22] | Bai, K. K.; Chen, F. L.; Guo, H. H. Chin. J. New Drug 2012, 21(13), 1536. (in Chinese) |
| [22] | (白锴凯, 陈芬玲, 郭养浩, 中国新药杂志, 2012, 21(13), 1536.) |
| [23] | Jiang, W.; Huang, R. Z.; Zhang, J.; Guo, T.; Zhang, M. T.; Huang, X. C.; Zhang, B.; Liao, Z. X.; Sun, J.; Wang, H. S. Bioorg. Chem. 2018, 79, 265. |
| [24] | Yu, Z.; Bai, K.-K.; Guo, Y.-H. Pharm. Biotechnol. 2010, 17(5), 382. (in Chinese) |
| [24] | (于舟, 白锴凯, 郭养浩, 药物生物技术, 2010, 17(5), 382.) |
| [25] | Dong, H.-Y.; Yang, X.; Xie, J.-J.; Xiang, L.; Li, Y.-F.; Ou, M.; Chi, T.; Liu, Z.; Yu, S.-H.; Gao, Y. Biochem. Pharmacol. 2015, 93(2), 151. |
| [26] | Hua, S. X.; Huang, R. Z.; Ye, M. Y.; Pan, Y. M.; Yao, G. Y.; Zhang, Y.; Wang, H. S. Eur. J. Med. Chem. 2015, 95(10), 435. |
| [27] | Huang, R. Z.; Hua, S. X.; Liao, Z. X.; Huang, X. C.; Wang, H. S. Med. Chem. Commun. 2017, 8(7), 1421. |
| [28] | Wang, J.-C.; Jiang, Z.; Xiang, L.-P.; Li, Y.-F.; Ou, M.-R.; Yang, X.; Shao, J.-W.; Lu, Y.-S.; Lin, L.-F.; Chen, J.-Z. Sci. Rep. 2014, 4, 5006. |
| [29] | Wiemann, J.; Heller, L.; Csuk, R. Bioorg. Med. Chem. Lett. 2016, 26(3), 907. |
| [30] | Yang, X.; Li, Y.; Jiang, W.; Ou, M.; Chen, Y.; Xu, Y.; Wu, Q.; Zheng, Q.; Wu, F.; Wang, L.; Zou, W.; Zhang, Y. J.; Shao, J. Chem. Biol. Drug Des. 2015, 86(6), 1397. |
| [31] | Bai, K. K.; Chen, F. L.; Zheng, Y. Q.; Guo, H. H. Chin. Pharm. J. 2012, 47(4), 265. (in Chinese) |
| [31] | (白锴凯, 陈芬玲, 郑允权, 郭养浩, 中国药学杂志, 2012, 47(4), 265.) |
| [32] | Shao, J. W.; Dai, Y. C.; Xue, J. P.; Wang, J. C.; Lin, F. P.; Guo, Y. H. Eur. J. Med. Chem. 2011, 46(7), 2652. |
| [33] | Popov, S. A.; Kornaukhova, L. M.; Shpatov, A. V.; Grigor'ev, I. A. Chem. Nat. Compd. 2016, 52(3), 555. |
| [34] | Wolfram, R. K.; Heller, L.; Csuk, R. Eur. J. Med. Chem. 2018, 152, 21. |
| [35] | Rashid, S.; Dar, B. A.; Majeed, R.; Hamid, A.; Bhat, B. A. Eur. J. Med. Chem. 2013, 66(66C), 238. |
| [36] | Yu, T.-T.; Li, L.; Cui, H.-B.; Meng, Y.-Q. Chem 2017, 80(11), 74. |
| [37] | Yan-Qiu, M.; Dan, L.; Zhong-Wei, B.; Ling-Li, C.; Hong-Ru, A. Acta Pharm. Sin. 2011, 46(5), 556. (in Chinese) |
| [37] | (孟艳秋, 刘丹, 白忠伟, 蔡伶俐, 艾宏儒, 药学学报, 2011, 46(5), 556.) |
| [38] | Meng, Y.-Q.; Song, Y.-L.; Yan, Z.-K.; Xia, Y. Molecules 2010, 15(6), 4033. |
| [39] | Xu, C.-D.; Meng, Y.-Q. J. Shenyang Univ. Chem. Technol. 2020, 34(1), 18. (in Chinese) |
| [39] | (徐川东, 孟艳秋, 沈阳化工大学学报, 2020, 34(1), 18.) |
| [40] | Meng, Y.-Q.; Xu, C.-D.; Yu, T.-T.; Li, W.; Li, Q.-W.; Li, X.-X. J. Asian Nat. Prod. Res. 2019, 24(4), 359. |
| [41] | Meng, Y.-Q.; Ding, Y.; Yang, Z.; Zhang, M. Drugs Clin. 2014, 29(2), 116. (in Chinese) |
| [41] | (孟艳秋, 丁一, 张萌, 杨哲, 现代药物与临床, 2014, 29(2), 116.) |
| [42] | Yao, Y.; Zhang, X.; Wang, Z.; Zheng, C.; Li, P.; Huang, C.; Tao, W.; Xiao, W.; Wang, Y.; Huang, L.; Yang, L. J. Ethnopharmacol. 2013, 150(2), 619. |
| [43] | Zhang, L.-F.; Meng, Y.-Q. J. Shenyang Univ. Chem. Technol. 2016, 30(2), 117. (in Chinese) |
| [43] | (张良锋, 孟艳秋, 沈阳化工大学学报, 2016, 30(2), 117.) |
| [44] | Li, A. L.; Hao, Y.; Wang, W. Y.; Liu, Q. S.; Gu, W. Int. J. Mol. Sci. 2020, 21(8), 2876. |
| [45] | Jin, X. Y.; Chen, H.; Li, D. D.; Li, A. L.; Gu, W. J. Enzyme Inhib. Med. Chem. 2019, 34(1), 955. |
| [46] | Liu, X.-Y.; Gao, X.-Q.; Jin, X.-J.; Zhao, C.-H.; Feng, Z.-H.; Sui, Y.; Zhao, L.-X.; Yan, X. Chin. J. Org. Chem. 2018, 38(12), 99. (in Chinese) |
| [46] | (刘新宇, 高雪琴, 金学军, 赵春晖, 冯中华, 隋悦, 赵龙铉, 阎欣, 有机化学, 2018, 38(12), 99.) |
| [47] | Zhang, T.; He, B.-E.; Yuan, H.; Feng, G.-L.; Chen, F.-L.; Wu, A.-Z.; Zhang, L.-L.; Lin, H.-R.; Zhuo, Z.-J.; Wang, T. Chem. Biodiversity 2019, 16(6), e1900111. |
| [48] | Wu, J.; Zhang, Z. H.; Zhang, L. H.; Jin, X. J.; Ma, J.; Piao, H. R. Bioorg. Med. Chem. Lett. 2019, 29(6), 853. |
| [49] | Fan, H.-T.; Geng, L.; Yang, F.; Dong, X.; He, D.; Zhang, Y. Eur. J. Med. Chem. 2019, 176, 61. |
| [50] | Pei, T.; Wang, Y.-Y. J. Chin. Pharm. Univ. 2017, 48(1), 31. (in Chinese) |
| [50] | (裴婷, 王玉英, 中国药科大学学报, 2017, 48(1), 31.) |
| [51] | Chen, Y.; Li, C.; Zheng, Y.; Gao, Y.; Hu, J.; Chen, H. Bioorg. Med. Chem. Lett. 2017, 27(4), 1007. |
| [52] | Li, W.; Zhang, H.; Nie, M.; Wang, W.; Liu, Z.; Chen, C.; Chen, H.; Liu, R.; Baloch, Z.; Ma, K. Oncol. Lett. 2018, 15(2), 2323. |
| [53] | Dar, B. A.; Lone, A. M.; Shah, W. A.; Qurishi, M. A. Eur. J. Med. Chem. 2016, 111, 26. |
| [54] | Finlay, H. J.; Honda, T.; Gribble, G. W.; Danielpour, D.; Benoit, N. E.; Suh, N.; Williams, C.; Sporn, M. B. Bioorg. Med. Chem. Lett. 1997, 7(13), 1769. |
| [55] | Gu, W.; Jin, X. Y.; Li, D. D.; Wang, S. F.; Tao, X. B.; Chen, H. Bioorg. Med. Chem. Lett. 2017, 27(17), 4128. |
| [56] | Gu, W.; Hao, Y.; Zhang, G.; Wang, S. F.; Miao, T. T.; Zhang, K. P. Bioorg. Med. Chem. Lett. 2015, 25(3), 554. |
| [57] | Lin, R. X.; Gong, L. L.; Fan, L. M.; Zhao, Z. K.; Yang, S. L. Int. J. Clin. Exp. Pathol. 2015, 8(2), 1427. |
| [58] | Mendes, V. I. S.; Bartholomeusz, G. A.; Ayres, M.; Gandhi, V.; Salvador, J. A. R. Eur. J. Med. Chem. 2016, 123, 317. |
| [59] | Sun, L.; Li, B.; Su, X.; Chen, G.; Li, Y.; Yu, L.; Li, L.; Wei, W. J. Med. Chem. 2017, 60(15), 6638. |
| [60] | Jin, X.-Y.; Chen, H.; Tao, X.-B.; Wang, S.; Gu, W. J. For. Eng. 2018, (1), 54. |
| [61] | Pan, H.-S.; Li, L.; Cui, H.-B.; Yang, L.-N.; Yu, T.-T.; Meng, Y.-Q. Acta Pharm. Sin. 2017, 52(12), 1890. (in Chinese) |
| [61] | (潘洪双, 李磊, 崔华博, 杨丽娜, 于婷婷, 孟艳秋, 药学学报, 2017, 52(12), 1890.) |
| [62] | Meng, Y.-Q.; Cao, J.; Tang, Y.; Lu, X.-Y.; Liu, L.-W. Chin. J. Org. Chem. 2016, 36(5), 1080. (in Chinese) |
| [62] | (孟艳秋, 曹佳, 汤义, 鹿学宇, 刘立伟, 有机化学, 2016, 36(5), 1080.) |
| [63] | Wang, Y.-Y.; Xiong, H. Strait. Pharm. J. 2017, 29(2), 268. (in Chinese) |
| [63] | (王玉英, 熊慧, 海峡药学, 2017, 29(2), 268.) |
| [64] | Wang, Y.-Y.; Xiong, H. Strait. Pharm. J. 2016, 28(12), 273. (in Chinese) |
| [64] | (王玉英, 熊慧, 海峡药学, 2016, 28(12), 273.) |
| [65] | Wei, Z.; Di, H.; Zhou, Y.; Zhang, Y.; Qiang, S.; Li, J. Y.; Hu, L. H.; Jia, L. Biochim. Biophys. Acta. Gen. Subj. 2006, 1760(10), 1505. |
| [66] | Guzmán-Ávila, R.; Flores-Morales, V.; Paoli, P.; Camici, G.; Ramírez-Espinosa, J. J.; Cerón-Romero, L.; Navarrete-Vázquez, G.; Hidalgo-Figueroa, S.; Yolanda, R. M.; Villalobos-Molina, R. Drug Dev. Res. 2018, 79(2). 70. |
| [67] | Kazmi, I.; Rahman, M.; Afzal, M.; Gupta, G.; Saleem, S.; Afzal, O.; Shaharyar, M. A.; Nautiyal, U.; Ahmed, S.; Anwar, F. Fitoterapia 2012, 83(1), 142. |
| [68] | Wu, P. P.; Zhang, K.; Lu, Y. J.; He, P.; Zhao, S. Q. Eur. J. Med. Chem. 2014, 80, 502. |
| [69] | Wu, P. P.; Zhang, B. J.; Cui, X. P.; Yang, Y.; Jiang, Z. Y.; Zhou, Z. H.; Zhong, Y. Y.; Mai, Y. Y.; Ouyang, Z.; Chen, H. S. Sci. Rep. 2017, 7, 45578. |
| [70] | Gao, Y.; Li, Z.; Xie, X.; Wang, C.; You, J.; Mo, F.; Jin, B.; Chen, J.; Shao, J.; Chen, H.; Jia, L. Eur. J. Pharm. Sci. 2015, 70, 55. |
| [71] | Chen, L.; Yang, X. S.; Yang, X.; Ye, L. H.; Hao, X. J. J. Chin. Pharm. Univer. 2010, 41(3), 222. (in Chinese) |
| [71] | (陈磊, 杨小生, 杨娟, 叶林虎, 郝小江, 中国药科大学学报, 2010, 41(3), 222.) |
| [72] | Zhang, L.; Dong, J.; Liu, J.; Zhang, L.; Kong, L.; Yao, H.; Sun, H. Med. Chem. 2013, 9(1), 118. |
| [73] | Chen, C.; Sun, R.; Sun, Y.; Chen, X.; Chen, D. Bioorg. Med. Chem. Lett. 2019, 30(2), 126824. |
| [74] | Wei, Z. Y.; Chi, K. Q.; Wang, K. S.; Wu, J.; Liu, L. P.; Piao, H. R. Bioorg. Med. Chem. Lett. 2018, 28(10), 1797. |
| [75] | Nelson, A. T.; Camelio, A. M.; Claussen, K. R.; Cho, J.; Tremmel, L.; Digiovanni, J.; Siegel, D. Bioorg. Med. Chem. Lett. 2015, 25(19), 4342. |
| [76] | Zhang, C.; Xu, S. H.; Ma, B. L.; Wang, W. W.; Yu, B. Y.; Zhang, J. Bioorg. Med. Chem. Lett. 2017, 27(11), 2575. |
| [77] | Li, C. H.; Yang, C. L.; Zhang, K.; Shi, W. Q.; Li, J. Z.; Li, Y.; Yin, S. F. Chin. J. Org. Chem. 2012, 32(1), 133. (in Chinese) |
| [77] | (李财虎, 杨聪玲, 张宽, 石万棋, 李剑忠, 李颖, 尹述凡, 有机化学, 2012, 32(1), 133.) |
| [78] | Tan, J.; Huang, W.; Chen, S.-L.; Yue, Y. Acta Pharm. Sin. 2016, 51(6), 938. (in Chinese) |
| [78] | (谭娟, 黄微, 陈善龙, 岳源, 药学学报, 2016, 51(6), 938.) |
| [79] | Li, C.; Chen, J.; Yuan, W.; Zhang, W.; Chen, H.; Tan, H. Iubmb. Life 2020, 72(4), 632. |
| [80] | Fu, H. J.; Zhou, Y. R.; Bao, B. H.; Jia, M. X.; Zhao, Y.; Zhang, L.; Li, J. X.; He, H. L.; Zhou, X. M. J. Med. Chem. 2014, 57(11), 4692. |
| [81] | Fu, H. J.; Zhao, Y.; Zhou, Y. R.; Bao, B. H.; Du, Y.; Li, J. X. Eur. J. Pharm. Sci. 2015, 76, 33. |
| [82] | Yu, S. G.; Zhang, C. J.; Xu, X. E.; Sun, J. H.; Zhang, L.; Yu, P. F. Int. J. Clin. Exp. Pathol. 2014, 8(4), 3681. |
| [83] | Kong, L.-B.; Li, S.-S.; Liao, Q.-J.; Zhang, Y.-N.; Sun, R.-N.; Zhu, X.-D.; Zhang, Q.-H.; Wang, J.; Wu, X.-Y.; Fang, X.-N. Antiviral Res. 2013, 98(1), 44. |
| [84] | Zhong, Y.; Dai, Z.; Xu, Y.; Teng, Y.; Wu, B. Eur. J. Pharm. Sci. 2012, 45(1-2), 110. |
| [85] | Li, S.-M.; Jia, X.-H.; Li, H.; Ye, Y.-L.; Song, G.-P. Bioorg. Med. Chem. Lett. 2020, 30(22), 127518. |
| [86] | Song, G.; Shen, X.; Li, S.; Li, Y.; Liu, Y.; Zheng, Y.; Lin, R.; Fan, J.; Ye, H.; Liu, S. Eur. J. Med. Chem. 2015, 93, 431. |
| [87] | Luan, T.; Jin, C. M.; Gong, G. H.; Quan, Z. S. J. Enzyme Inhib. Med. Chem. 2019, 34(1), 761. |
| [88] | Sifaoui, I.; Rodríguez-Expósito, R. L.; Reyes-Batlle, M.; Rizo- Liendo, A.; Piero, J. E.; Bazzocchi, I. L.; Lorenzo-Morales, J.; Jiménez, I. A. Pathogens 2019, 8(3), 130. |
| [89] | Wang, P. Y.; Xiang, M.; Luo, M.; Liu, H. W.; Zhou, X.; Wu, Z. B.; Liu, L. W.; Li, Z.; Yang, S. Pest. Manage. Sci. 2020, 76(8), 2746. |
| [90] | Cunha, L. C. S.; Silva, M. L. a. E.; Furtado, N. a. X. C.; Vinholis, A. H. C.; Martins, C. H. G.; Filho, A. A. D. S.; Cunha, W. R. Z. Naturforsch., C: J. Biosci. 2007, 62(9-10), 668. |
| [91] | Do Nascimento, P. G. G.; Lemos, T. L. G.; Bizerra, A. M. C.; Arriaga, Â. M. C.; Ferreira, D. A.; Santiago, G. M. P.; Braz-Filho, R.; Costa, J. G. M. Molecules 2014, 19(1), 1317. |
| [92] | Dwivedi, G. R.; Maurya, A.; Yadav, D. K.; Khan, F.; Darokar, M. P.; Srivastava, S. K. Chem. Biol. Drug Des. 2015, 86(3), 272. |
| [93] | Jabeen, M.; Ahmad, S.; Shahid, K.; Sadiq, A.; Rashid, U. Front. Chem. 2018, 6, 55. |
| [94] | Huang, L. R.; Luo, H.; Yang, X. S.; Lei, C.; Zhang, J. X.; Wang, D. P.; Hao, X. J. Med. Chem. Res. 2014, 23(11), 4631. |
| [95] | Meng, Y.-C.; Zhan, J.-H.; Xiao, S.-P.; Tan, Y.; Liao, M.-F.; Zhang, Y.-L.; Li, L.; Pei, G. J. Hunan Univ. Chin. Med. 2017, 37(5), 493. (in Chinese) |
| [95] | (孟英才, 詹济华, 肖水平, 谭洋, 廖美芳, 张雨林, 李玲, 裴刚, 湖南中医药大学学报, 2017, 37(5), 493.) |
| [96] | Gnoatto, S.; Dalla-Vechia, L., Cl; Dassonville-Klimpt, A.; Da-Nascimento, S.; Mossalayi, D.; Guillon, J.; Gosmann, G.; Sonnet, P. J. Enzyme Inhib. Med. Chem. 2008, 23(5), 604. |
| [97] | Innocente, A. M.; Silva, G. N. S.; Laura Nogueira, C.; Moraes, M. S.; Myna, N.; Pascal, S.; Grace, G.; Garcia, C. R. S.; Gnoatto, S. C. B. Molecules 2012, 17(10), 12003. |
| [98] | Silva, G. N. D.; Maria, N. R.; Schuck, D. C.; Cruz, L. N.; Moraes, M. S. D.; Nakabashi, M.; Graebin, C.; Gosmann, G.; Garcia, C. R.; Gnoatto, S. C. Malar. J. 2013, 12(1), 89. |
| [99] | Cargnin, S. T.; Staudt, A. F.; Medeiros, P.; Sol, D. D. M. S.; Santos, A. P. D. a. D.; Zanchi, F. B.; Gosmann, G.; Puyet, A.; Teles, C. B. G.; Gnoatto, S. B. Bioorg. Med. Chem. Lett. 2018, 28(3), 265. |
| [100] | Bitencourt, F. G.; Vieira, P. D. B.; Meirelles, L. C.; Rigo, G. V.; Silva, E. F. D.; Gnoatto, S. C. B.; Tasca, T. Parasitol. Res. 2018, 117(5), 1. |
| [101] | Loesche, A.; Köwitsch, A.; Lucas, S. D.; Al-Halabi, Z.; Sippl, W., Al-Harrasi, A; Csuk, R. Bioorg. Chem. 2019, 85, 23. |
| [102] | Imran, K.; Muhammad, A.; Gaurav, G.; Firoz, A. CNS Neurosci. Ther. 2012, 18(9), 799. |
| [103] | Kazmi, I.; Gupta, G.; Afzal, M.; Anwar, F. Asian Pac. J. Trop. Dis. 2012, 2(Suppl. 1), S453. |
| [104] | Imran, K.; Gaurav, G.; Muhammad, A.; Mahfoozur, R.; Firoz, A. CNS Neurosci. Ther. 2012, 18 (8), 707. |
/
| 〈 |
|
〉 |