

无金属参与的电化学促进2,2,6,6-四甲基哌啶-氮-氧化物(TEMPO)介导的环胺α-氰化和膦酰化反应
收稿日期: 2021-03-26
修回日期: 2021-04-26
网络出版日期: 2021-05-14
基金资助
国家自然科学基金(21772222); 国家自然科学基金(21821002); 广东省教育厅基金(2017KSYS010); 广东省教育厅基金(2017KZDXM084); 广东省教育厅基金(2019KZDZX2003); 广东省教育厅基金(2019KZDXM035)
Electrochemical 2,2,6,6-tetramethylpiperidinyl-N-oxyl (TEMPO)-Mediated α-Cyanation and Phosphonylation of Cyclic Amines with Metal-Free Conditions
Received date: 2021-03-26
Revised date: 2021-04-26
Online published: 2021-05-14
Supported by
National Natural Science Foundation of China(21772222); National Natural Science Foundation of China(21821002); Department of Education of Guangdong Province(2017KSYS010); Department of Education of Guangdong Province(2017KZDXM084); Department of Education of Guangdong Province(2019KZDZX2003); Department of Education of Guangdong Province(2019KZDXM035)
高君青 , 翁信军 , 马聪 , 徐学涛 , 方萍 , 梅天胜 . 无金属参与的电化学促进2,2,6,6-四甲基哌啶-氮-氧化物(TEMPO)介导的环胺α-氰化和膦酰化反应[J]. 有机化学, 2021 , 41(8) : 3223 -3234 . DOI: 10.6023/cjoc202103049
Metal-free electrochemical oxidation cyanation and phosphonylation reactions had been developed, in which 2,2,6,6-tetramethylpiperidinyl-N-oxyl (TEMPO) reduced the electrode potential of substrate and avoided over oxidation of some electron rich aromatic amines under electrochemical conditions. This protocol had good functional group compatibility, which made it to be a practical and efficient method to synthesize α-aminonitriles and α-amino phosphonates under mild conditions. Preliminary study indicated that the formation of the product was through the Shono oxidation of imine species.
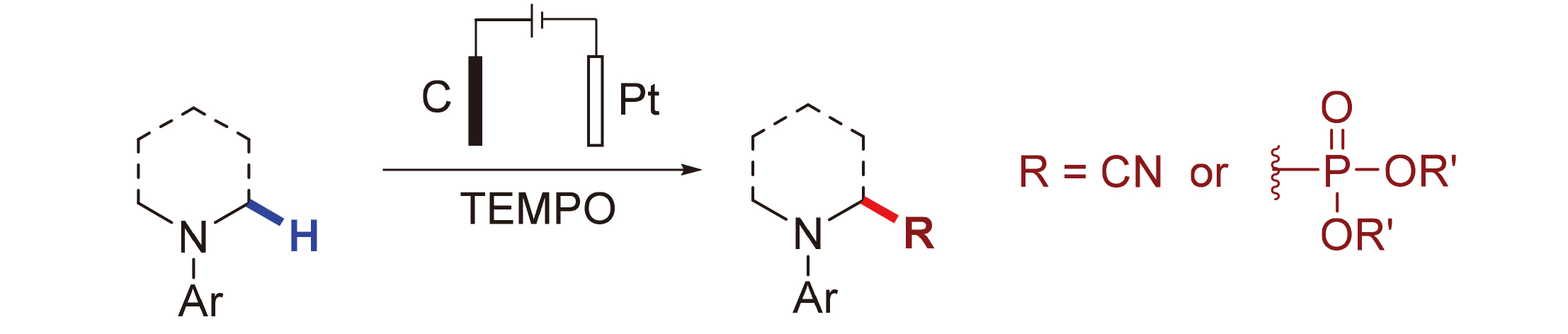
Key words: electrochemistry; Shono oxidation; cyanation; phosphonylation
| [1] | (a) Weinstock, L. M.; Davis, P.; Handelsman, B.; Tull, R. J. J. Org. Chem. 1967, 32, 2823. |
| [1] | (b) Matier, W. L.; Owens, D. A.; Comer, W. T.; Deitchman, D.; Ferguson, H. C. Seidehamel, R. J.; Young, J. R. J. Med. Chem. 1973, 16, 901. |
| [1] | (c) Enders, D.; Shilvock, J. P. Chem. Soc. Rev. 2000, 29, 359. |
| [2] | (a) Shono, T.; Matsumura, Y.; Uchida, K.; Tsubata, K.; Makino, A. J. Org. Chem. 1984, 49, 300. |
| [2] | (b) Myers, E. L.; de Vries, J. G.; Aggarwal, V. K. Angew. Chem. Int. Ed. 2007, 46, 1893. |
| [2] | (c) Kabeshov, M. A.; Musio, B.; Murray, P. R.; Browne, D. L.; Ley, S. V. Org. Lett. 2014, 16, 4618. |
| [3] | (a) Moeller, K. D.; Wong, P. L. Bioorg. Med. Chem. Lett. 1992, 2, 739. |
| [3] | (b) Frankowski, K. J.; Liu, R.; Milligan, G. L.; Moeller, K. D.; Aube, J. Angew. Chem. Int. Ed. 2015, 54, 10555. |
| [4] | Myers, E. L.; de Vries, J. G.; Aggarwal, V. K. Angew. Chem. 2007, 119, 1925. |
| [5] | (a) Rappoport, Z. The Chemistry of the Cyano Group, Interscience Publishers, London, 1970. |
| [5] | (b) Larock, R. C. Comprehensive Organic Transformations: A Guide to Functional Group Preparations, VCH, New York, 1989. |
| [6] | For selected examples on Fe-catalyzed, see: |
| [6] | (a) Singhal, S.; Jain, S. L.; Sain, B. Adv. Synth. Catal. 2010, 352, 1338. |
| [6] | (b) Liu, P.; Liu, Y.; Wong, E. L-M.; Xiang, S.; Che, C.-M. Chem. Sci. 2011, 2, 2187. |
| [6] | (c) Sun, C. L.; Li, B. J.; Shi, Z. J. Chem. Rev. 2011, 111, 1293. |
| [6] | (d) Wagner, A.; Han, W.; Mayer, P.; Ofial, A. R. Adv. Synth. Catal. 2013, 355, 3058. |
| [6] | (e) Patil, M.; Kapdi, A. R.; Kumar, A. V. RSC Adv. 2015, 5, 54505. |
| [6] | (f) Wagner, A.; Hampel, N.; Zipse, H.; Ofial, A. R. Org. Lett. 2015, 17, 4770. |
| [6] | (g) Nauth, A. M.; Otto, N.; Opatz, T. Adv. Synth. Catal. 2015, 357, 3424. |
| [6] | (h) Panwar, V.; Kumar, P.; Bansal, A.; Ray, S. S.; Jain, S. L. Appl. Catal., 2015, 498, 25. |
| [6] | (i) Zhang, L.; Gu, X.; Lu, P.; Wang, Y. Tetrahedron 2016, 72, 2359. |
| [6] | (j) Huang, M.; Deng, Q.; Gao, Q.; Shi, J.; Zhang, X.; Xiong, Y. Asian J. Org. Chem. 2018, 7, 404. |
| [7] | For selected examples on Cu-catalyzed, see: |
| [7] | (a) Li, Z.; Li, C.-J. Eur. J. Org. Chem., 2005, 2005, 3173. |
| [7] | (b) Han, W.; Ofial, A. R. Chem. Commun. 2009, 5024. |
| [7] | (c) Ogibin, Y. N.; Elinson, M. N.; Nikishin, G. I. Russ. Chem. Rev. 2009, 78, 89. |
| [7] | (d) Boess, E.; Schmitz, C.; Klussmann, M. J. Am. Chem. Soc. 2012, 134, 5317. |
| [7] | (e) Zhang, G.; Ma, Y.; Cheng, G.; Liu, D.; Wang, R. Org. Lett. 2014, 16, 656. |
| [7] | (f) Fandrick, D. R.; Hart, C. A.; Okafor, I. S.; Mercadante, M. A.; Sanyal, S.; Masters, J. T.; Sarvestani, M.; Fandrick, K. R.; Stockdill, J. L.; Grinberg, N.; Gonnella, N.; Lee, H.; Senanayake, C. H. Org. Lett. 2016, 18, 6192. |
| [7] | (g) Liu, Y.; Wang, C.; Xue, D.; Xiao, M.; Li, C.; Xiao, J. Chem.- Eur. J. 2017, 23, 3051. |
| [8] | For selected examples on Ir-catalyzed, see: a Rueping, |
| [8] | (a) Rueping, |
| [8] | (b) Verma, D.; Verma, S.; Sinha, A. K.; Jain, S. L. ChemPlusChem 2013, 78, 860. |
| [8] | (c) Ide, T.; Shimizu, K.; Egami, H.; Hamashima, Y. Tetrahedron Lett. 2018, 59, 3258. |
| [8] | (d) Yilmaz, O.; Oderinde, M. S.; Emmert, M. H. J. Org. Chem. 2018, 83, 11089. |
| [9] | For selected examples on Ru-catalyzed, see: |
| [9] | (a) Murahashi, S. I.; Komiya, N.; Terai, H.; Nakae, T. J. Am. Chem. Soc. 2003, 125, 15312. |
| [9] | (b) North, M. Angew. Chem. Int. Ed. 2004, 43, 4126. |
| [9] | (c) Murahashi, S.; Komiya, N.; Terai, H. Angew. Chem. Int. Ed. 2005, 44, 6931. |
| [9] | (d) Murahashi, S. I.; Nakae, T.; Terai, H.; Komiya, N. J. Am. Chem. Soc. 2008, 130, 11005. |
| [9] | (e) Verma, S.; Jain, S. L.; Sain, B. ChemCatChem 2011, 3, 1329. |
| [9] | (f) Verma, S.; Jain, S. L.; Sain, B. Catal. Lett. 2011, 141, 882. |
| [9] | (g) Schümperli, M. T.; Hammond, C.; Hermans, I. ACS Catal. 2012, 2, 1108. |
| [9] | (h) Panwar, V.; Ray, S. S.; Jain, S. L. Tetrahedron Lett. 2015, 56, 4184. |
| [10] | For selected examples on Ti-catalyzed, see: |
| [10] | (a) Rueping, M.; Zoller, J.; Fabry, D. C.; Poscharny, K.; Koenigs, R. M.; Weirich, T. E.; Mayer, J. Chem.-Eur. J. 2012, 18, 3478. |
| [10] | (b) Kumar, P.; Varma, S.; Jain, S. L. J. Mater. Chem. A 2014, 2, 4514. |
| [10] | (c) Nauth, A. M.; Schechtel, E.; Doren, R.; Tremel, W.; Opatz, T. J. Am. Chem. Soc. 2018, 140, 14169. |
| [11] | For selected examples on other metal-catalyzed, see: |
| [11] | (a) Singhal, S.; Jain, S. L.; Sain, B. Chem. Commun. 2009, 2371. |
| [11] | (b) Zhang, Y.; Peng, H.; Zhang, M.; Cheng, Y.; Zhu, C. Chem. Commun. 2011, 47, 2354. |
| [11] | (c) Sakai, N.; Mutsuro, A.; Ikeda, R.; Konakahara, T. Synlett 2013, 24, 1283. |
| [11] | (d) Lin, A.; Peng, H.; Abdukader, A.; Zhu, C. Eur. J. Org. Chem. 2013, 2013, 7286. |
| [11] | (e) Yang, W.; Wei, L.; Yi, F.; Cai, M. Tetrahedron 2016, 72, 4059. |
| [11] | (f) Ping, Y.; Ding, Q.; Peng, Y. ACS Catal. 2016, 6, 5989. |
| [11] | (g) Liang, W.; Zhang, T.; Liu, Y.; Huang, Y.; Liu, Z.; Liu, Y.; Yang, B.; Zhou, X.; Zhang, J. ChemSusChem 2018, 11, 3586. |
| [11] | (h) Patil, M. R.; Dedhia, N. P.; Kapdi, A. R.; Kumar, A. V. J. Org. Chem. 2018, 83, 4477. |
| [11] | (i) Casado-Sanchez, A.; Uygur, M.; Gonzalez-Munoz, D.; Aguilar-Galindo, F.; Nova-Fernandez, J. L.; Arranz-Plaza, J.; Diaz-Tendero, S.; Cabrera, S.; Mancheno, O. G.; Aleman, J. J. Org. Chem. 2019, 84, 6437. |
| [12] | For selected examples on non-metal catalyzed, such as: AIBN, TBAI, TBHP, TBP, see: |
| [12] | (a) Liu, L. H.; Wang, Z. K.; Fu, X. F.; Yan, C. H. Org. Lett. 2012, 14, 5692. |
| [12] | (b) Zhang, C.; Liu, C.; Shao, Y.; Bao, X.; Wan, X. Chem.-Eur. J. 2013, 19, 17917. |
| [12] | (c) Wang, H.; Shao, Y.; Zheng, H.; Wang, H.; Cheng, J.; Wan, X. Chem.-Eur. J. 2015, 21, 18333. |
| [12] | (d) Zhang, Z.; Gu, K.; Bao, Z.; Xing, H.; Yang, Q.; Ren, Q. Tetrahedron 2017, 73, 3118. |
| [12] | (e) Liu, P. Y.; Zhang, C.; Zhao, S. C.; Yu, F.; Li, F.; He, Y. P. J. Org. Chem. 2017, 82, 12786. |
| [12] | (f) Sun, M. X.; Wang, Y. F.; Xu, B. H.; Ma, X. Q.; Zhang, S. J. Org. Biomol. Chem. 2018, 16, 19717. |
| [12] | (g) Ullah, B.; Zhou, Y.; Chen, J.; Bao, Z.; Yang, Y.; Yang, Q.; Ren, Q.; Zhang, Z. Tetrahedron Lett. 2019, 60, 348. |
| [13] | For selected examples on non-metal catalyzed, such as: PhIOAc2 and AcOH, see: |
| [13] | (a) Shu, X. Z.; Xia, X. F.; Yang, Y. F.; Ji, K. G.; Liu, X. Y.; Liang, Y. M. J. Org. Chem. 2009, 74, 7464. |
| [13] | (b) Ueda, H.; Yoshida, K.; Tokuyama, H. Org. Lett. 2014, 16, 4194. |
| [14] | For selected examples on other non-metal catalyzed, see: |
| [14] | (a) Rueping, M.; Zhu, S.; Koenigs, R. M. Chem. Commun. 2011, 47, 8679. |
| [14] | (b) Allen, J. M.; Lambert, T. H. J. Am. Chem. Soc. 2011, 133, 1260. |
| [14] | (c) Ushakov, D. B.; Gilmore, K.; Kopetzki, D.; McQuade, D. T.; Seeberger, P. H. Angew. Chem. Int. Ed. 2014, 53, 557. |
| [14] | (d) Kabeshov, M. A.; Musio, B.; Murray, P. R.; Browne, D. L.; Ley, S. V. Org. Lett. 2014, 16, 4618. |
| [14] | (e) Shen, H.; Zhang, X.; Liu, Q.; Pan, J.; Hu, W.; Xiong, Y.; Zhu, X. Tetrahedron Lett. 2015, 56, 5628. |
| [14] | (f) Orejarena Pacheco, J.C.; Lipp,, A.; Nauth,, A. M.; Acke,, F.; Dietz,, J. P.; Opatz,, T. Chem.-Eur. J. 2016, 22, 5409. |
| [14] | (g) Fu, N.; Li, L.; Yang, Q.; Luo, S. Org. Lett. 2017, 19, 2122. |
| [14] | (h) Chen, W.; Ma, L.; Paul, A.; Seidel, D. Nat. Chem. 2018, 10, 165. |
| [14] | (i) Periasamy, M.; Rao, G. Tetrahedron 2018, 74, 7209. |
| [15] | For selected examples on other mediated catalyzed, see: |
| [15] | (a) Wagner, A.; Ofial, A. R. J. Org. Chem. 2015, 80, 2848. |
| [15] | (b) Wang, F.; Rafiee, M.; Stahl, S. S. Angew. Chem. Int. Ed. 2018, 57, 6686. |
| [15] | (c) Lennox, A. J. J.; Goes, S. L.; Webster, M. P.; Koolman, H. F.; Djuric, S. W.; Stahl, S. S. J. Am. Chem. Soc. 2018, 140, 11227. |
| [15] | (d) Nutting, J. E.; Rafiee, M.; Stahl, S. S. Chem. Rev. 2018, 118, 4834. |
| [16] | (a) To, W. P.; Tong, G. S.; Lu, W.; Ma, C.; Liu, J.; Chow, A. L.; Che, C. M. Angew. Chem. Int. Ed. 2012, 51, 2654. |
| [16] | (b) Ushakov, D. B.; Gilmore, K.; Kopetzki, D.; McQuade, D. T.; Seeberger, P. H. Angew. Chem. Int. Ed. 2014, 53, 557. |
| [16] | (c) Orejarena Pacheco, J.C.; Lipp,, A.; Nauth,, A. M.; Acke,, F.; Dietz,, J. P.; Opatz,, T. Chem.-Eur. J. 2016, 22, 5409. |
| [16] | (d) Nauth, A. M.; Lipp, A.; Lipp, B.; Opatz, T. Eur. J. Org. Chem. 2017, 2099. |
| [16] | (e) Nauth, A. M.; Orejarena Pacheco, J. C.; Pusch, S.; Opatz, T. Eur. J. Org. Chem. 2017, 6966. |
| [16] | (f) Nauth, A. M.; Schechtel, E.; Doren, R.; Tremel, W.; Opatz, T. J. Am. Chem. Soc. 2018, 140, 14169. |
| [16] | (g) Yi, B.; Yan, N.; Yi, N.; Xie, Y.; Wen, X.; Au, C.-T.; Lan, D. Chem.-Asian J. 2020, 15, 4302. |
| [17] | For selected reviews on electrochemical C—H functionalization, see: |
| [17] | (a) Yang, Q.-L.; Fang, P.; Mei, T.-S. Chin. J. Chem. 2018, 36, 338. |
| [17] | (b) Ma, C.; Fang, P.; Mei, T.-S. ACS. Catal. 2018, 8, 7179. |
| [17] | (c) Yang, F.; Zhang, H.; Liu, X.; Wang, B.; Ackermann, L. Chin. J. Org. Chem. 2019, 39, 59. |
| [17] | (d) Meng, Z.-Y.; Feng, C.-T.; Xu, K. Chin. J. Org. Chem. 2021, 41, 2535. (in Chinese) |
| [17] | (蒙泽银, 冯承涛, 徐坤, 有机化学, 2021, 41, 2535.) |
| [18] | For selected reviews or highlights on electrochemistry, see: |
| [18] | (a) Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230. |
| [18] | (b) Tang, S.; Liu, Y.; Lei, A. Chem 2018, 4, 27. |
| [18] | (c) Ma, H. X.; Mei, T. S. Chin. J. Org. Chem. 2020, 40, 3982. (in Chinese) |
| [18] | (马红星, 梅天胜, 有机化学, 2020, 40, 3982.) |
| [18] | (d) Jiang, Y.-Y.; Zeng, C.-C. Chin. J. Org. Chem. 2020, 40, 2999. (in Chinese) |
| [18] | (蒋洋叶, 曾程初, 有机化学, 2020, 40, 2999.) |
| [18] | (e) Ye, Z. H.; Zhang, F. Z. Chin. J. Org. Chem. 2020, 40, 241. (in Chinese) |
| [18] | (叶增辉, 张逢质, 有机化学, 2020, 40, 241.) |
| [18] | (f) Wang, X. Y.; Xu, X. T.; Wang, Z. H.; Fang, P.; Mei, T. S. Chin. J. Org. Chem. 2020, 40, 3738. (in Chinese) |
| [18] | (王向阳, 徐学涛, 王振华, 方萍, 梅天胜, 有机化学, 2020, 40, 3738.) |
| [19] | (a) Shono, T.; Matsumura, Y.; Tsubata, K. J. Am. Chem. Soc. 1981, 103, 1172. |
| [19] | (b) Shono, T.; Matsumura, Y.; Uchida, K.; Tsubata, K.; Makino, A. J. Org. Chem. 1984, 49, 300. |
| [20] | Gao, P. S.; Weng, X. J.; Wang, Z. H.; Zheng, C.; Sun, B.; Chen, Z. H.; You, S. L.; Mei, T. S. Angew. Chem. Int. Ed. 2020, 59, 15254. |
| [21] | (a) Steckhan, V. E. Angew. Chem. 1986, 98, 681. |
| [21] | (b) Ogibin, Y. N.; Elinson, M. N.; Nikishin, G. I. Russ. Chem. Rev. 2009, 78, 89. |
| [21] | (c) Francke, R.; Little, R. D. Chem. Soc. Rev. 2014, 43, 2492. |
| [22] | (a) Quint, V.; Chouchène, N.; Askri, M.; Lalevée, J.; Gaumont, A.-C.; Lakhdar, S. Org. Chem. Front. 2019, 6, 41. |
| [22] | (b) Huang, M.; Dai, J.; Cheng, X.; Ding, M. Org. Lett. 2019, 21, 7759. |
| [23] | Rao, G. A.; Periasamy, M. Synthesis 2018, 50, 617. |
| [24] | Yi, B.; Yan, N.; Yi, N.; Xie, Y.; Wen, X.; Au, C.-T.; Lan, D. RSC Adv. 2019, 9, 29721. |
| [25] | Wang, H. L.; Li, X. C.; Wu, F.; Wan, B. S. Tetrahedron Lett. 2012, 53, 681. |
| [26] | Lin, B. Z.; Shi, S. S.; Lin, R. C.; Cui, Y. Q.; Fang, M. J.; Tang, G.; Zhao, Y. F. J. Org. Chem. 2018, 83, 6754. |
| [27] | Beke, D.; Bárczai, B. M.; Fcze, L. Chem. Ber. 1962, 95, 1054. |
/
| 〈 |
|
〉 |