

手性单磷酰保护二胺催化二乙基锌还原三氟甲基酮
收稿日期: 2021-02-26
修回日期: 2021-04-02
网络出版日期: 2021-06-08
基金资助
江苏高校2019年“蓝色工程”优秀教学团队(2019-69)
Reduction of Trifluoromethyl Ketones with Diethyl Zinc Catalyzed by Chiral Monophosphoryl Protected Diamine
Received date: 2021-02-26
Revised date: 2021-04-02
Online published: 2021-06-08
Supported by
Outstanding Teaching Team of 2019 “Blue Project” in Jiangsu Colleges and Universities(2019-69)
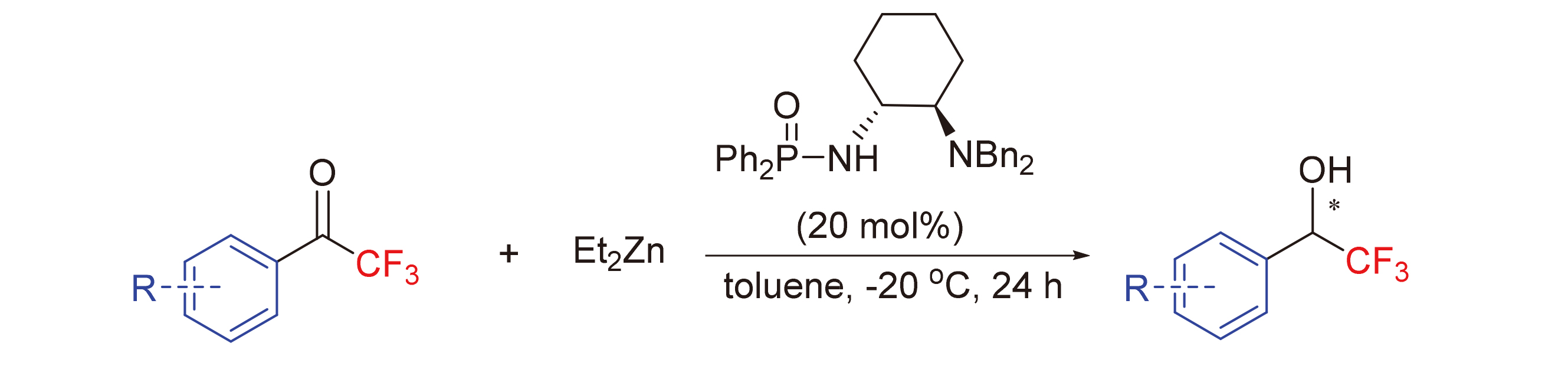
手性三氟甲基类化合物拥有重要生理活性, 为了获得该类化合物, 以磷酰胺为配体, 乙基锌、三氟甲基芳香醛及其衍生物为底物合成出一系列的三氟甲基类化合物, 所使用的原料廉价易得, 催化效率较高. 在最优条件下, 可以高收率、高ee值地合成相应的手性三氟甲基类化合物. 尽管配体使用量较高, 但其可回收利用. 最后, 对可能的反应机理进行了合理推测, 认为反应的高立体选择性主要归因于催化过程中形成的两个六元环过渡态及空间位阻作用.
关键词: 磷酰胺配体; 不对称催化; 手性三氟甲基类化合物
王雪 . 手性单磷酰保护二胺催化二乙基锌还原三氟甲基酮[J]. 有机化学, 2021 , 41(7) : 2693 -2699 . DOI: 10.6023/cjoc202102048
In order to obtain trifluoromethylated organic compounds with important biological activity, using phosphoramide as catalyst, diethylzinc and trifluoromethyl aromatic aldehyde as reactants, chiral trifluoromethylated organic compounds were synthesized through catalytic asymmetricβ-H transfer reduction reaction. The raw materials are cheap and easy to obtain, and the catalytic efficiency is high. The yield and ee value can be guaranteed to be highly and simultaneously under the optimized reaction conditions. Despite the large amount of catalyst, the ligand is very convenient to recycle and reuse in this system. At the same time, the reaction mechanism was speculated, and it was considered that the high stereoselectivity of the reaction was due to the formation of two six membered ring transition states and steric hindrance.

| [1] | (a) Xu,W. Y.; Feng,Y. S. Chem. J. Chin. Univ. 2020, 41,1567 (in Chinese). |
| [1] | ( 徐文艺, 冯乙巳, 高等学校化学学报, 2020, 41,1567.) |
| [1] | (b) Hu,Y. L.; Yang,T. Y.; Deng,Z. B.; Wang,K. H.; Li,P. F.; Huang,D. F.; Su,Y. P. J. Org. Chem. 2020, 85,12304. |
| [1] | (c) Xie,Q. Q.; Hu,J. B. Chin. J. Chem. 2020, 38,202. |
| [1] | (d) Schlosser, M. Angew. Chem.,Int. Ed. 2006, 45,5432. |
| [1] | (e) Muller, K.; Faeh, C.; Diederich, F. Science 2007, 317,1881. |
| [1] | (f) Purser, S.; Moore,P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37,320. |
| [1] | (g) Kirk,K. L. Org. Process Res. Dev. 2008, 12,305. |
| [2] | Shibatomi, K.; Narayama, A.; Abe Y.; Iwasa, S. Chem. Commun. 2012, 48,7380. |
| [3] | Endeshaw,M. M.; Li, C.; Leon,J. D.; Yao, N.; Latibeaudiere, K.; Premalatha, K.; Morrissette, N.; Werbovetz, K. Bioorg. Med. Chem. Lett. 2010, 20,5179. |
| [4] | Plosker,G. L.; Perry,C. M.; Goa,K. L. Pharmacoeconomics 2001, 19,421. |
| [5] | Ikeda, M.; Takahashi, K.; Dan, A.; Koyama, K.; Kubota, K.; Tanaka, T.; Hayashi, M. Bioorg. Med. Chem. 2000, 8,2157. |
| [6] | Bednarz,M. S.; Paul,S. D.; Kanamarlapudi,R. C.; Perlberg, A.; Zhang,H. M.. US 8653094 2014. |
| [7] | (a) Ohkuma, T.; Koizumi, M.; Doucet, H.; Pham, T.; Kozawa, M.; Murata, K.; Katayama, E.; Yokozawa, T.; Ikariya, T.; Noyori, R. J. Am. Chem. Soc. 1998, 120,13529. |
| [7] | (b) Kuroki, Y.; Asada, D.; Iseki, K. Tetrahedron Lett. 2000, 41,9853. |
| [7] | (c) Kuroki, Y.; Sakamaki, Y.; Iseki, K. Org. Lett. 2001, 3,457. |
| [7] | (d) Sterk, D.; Stephan, M.; Mohar,B. S.; Szollosi, G.; Bartok, M. Appl. Catal. A: Gen. 2009, 362,178. |
| [7] | (f) Pereniguez, R.; Santarossa, G.; Mallat, T.; Baiker, A. J. Mol. Catal. A: Chem. 2012, 365,39. |
| [8] | (a) Gaspar, J.; Guerrero, A. Tetrahedron: Asymmetry 1995, 6,231. |
| [8] | (b) Nakamura, K.; Matsuda, T.; Itoh, T.; Ohno, A. Tetrahedron Lett. 1996, 37,5727. |
| [8] | (c) Nakamura, K.; Matsuda, T.; Shimizu, M.; Fujisawa, T. Tetrahedron 1998, 54,8393. |
| [8] | (d) Inoue, K.; Makino, Y.; Itoh, N. Tetrahedron: Asymmetry 2005, 16,2539. |
| [8] | (e) Borzecka, W.; Lavandera, I.; Gotor, V. J. Org. Chem. 2013, 78,7312. |
| [9] | (a) Yamaguch, S.; Mosher,H. S. J. Org. Chem. 1973, 38,1870. |
| [9] | (b) Pirkle,W. H.; Sikkenga,D. L.; Pavlin,M. S. J. Org. Chem. 1977, 42,384. |
| [9] | (c) Hawkins,J. M.; Sharpless,K. B. J. Org. Chem. 1984, 49,3861. |
| [9] | (d) Chong,J. M.; Mar,E. K. J. Org. Chem. 1991, 56,893. |
| [10] | (a) Stepanenko, V.; De,J. M.; Correa, W.; Guzman, I.; Vazquez, C.; Cruz, W.; Ortiz-Marciales, M.; Barnes,C. L. Tetrahedron Lett. 2007, 48,5799. |
| [10] | (b) Korenaga, T.; Nomura, K.; Onoue, K.; Sakai, T. Chem. Commun. 2010, 46,8624. |
| [10] | (c) Kawanami, Y.; Hoshino, K.; Tsunoi, W. Tetrahedron: Asymmetry 2011, 22,1464. |
| [10] | (d) Turgut, Y.; Azizoglu, M.; Erdogan, A.; Arslan, N.; Hosgoren, H. Tetrahedron: Asymmetry 2013, 24,853. |
| [10] | (e) Harauchi, Y.; Takakura, C.; Furumoto, T.; Yanagita,R. C.; Kawanami, Y. Tetrahedron: Asymmetry 2015, 26,333. |
| [11] | (a) Nasipuri, D.; Bhattacharya,P. K. J. Chem. Soc.,Perkin Trans. 1 1977,576. |
| [11] | (b) Morrison,J. D.; Tomaszewski,J. E.; Mosher,H. S.; Dale, J.; Miller, D.; Elsenbaumer,R. L. J. Am. Chem. Soc. 1977, 99,3167. |
| [11] | (c) Yong,K. H.; Chong,J. M. Org. Lett. 2002, 4,4139. |
| [12] | Sasaki, S.; Yamauchi, T.; Kubo, H.; Kanai, M.; Ishii, A.; Higashiyama, K. Tetrahedron Lett. 2005, 46,1497. |
| [13] | (a) Yearick, K.; Wolf, C. Org. Lett. 2008, 10,3915. |
| [13] | (b) Genov, M.; Martinez-Ilarduya,J. M.; Calvillo-Barahona, M.; Espinet, P. Organometallic 2010, 29,6402. |
| [13] | (c) Calvillo-Barahona, M.; Cordovilla, C.; Genov,M. N.; Martinez-Ilarduya,J. M.; Espinet, P. Dalton Trans. 2013, 42,14576. |
| [13] | (d) Calvillo-Barahona, M.; Casares,J. A.; Cordovilla, C.; Genov,M. N.; Martinez-Ilarduya,J. M.; Espinet, P. Chem.-Eur. J. 2014, 20,14800. |
| [14] | Huang, H.; Zong, H.; Bian,G. L.; Song, L. Tetrahedron: Asymmetry 2015, 26,835. |
| [15] | (a) Forni, A.; Moretti, I.; Prati, F.; Torre, G. Tetrahedron 1994, 50,11995. |
| [15] | (b) Asao, N.; Asano, T.; Yamamoto, Y. Angew. Chem.,Int. Ed. 2001, 40,3206. |
| [15] | (c) Hess, R.; Diezi, S.; Mallat, T.; Baiker, A. Tetrahedron: Asymmetry 2004, 15,251. |
| [15] | (d) Diezi, S.; Hess, M.; Orglmeister, E.; Mallat, T.; Baiker, A. Catal. Lett. 2005, 102,121. |
| [15] | (e) Grau,B. T.; Devine,P. N.; DiMichele,L. N.; Kosjek, B. Org. Lett. 2007, 9,4951. |
| [16] | (a) Huang,H. Y.; Zong, H.; Bian,G. L.; Song, L. J. Org. Chem. 2012, 77,10427. |
| [16] | (b) Zong, H.; Huang,H. Y.; Bian,G. L.; Song, L. Tetrahedron Lett. 2013, 54,2722. |
| [16] | (c) Shen, B.; Huang,H. Y.; Bian,G. L.; Zong, H.; Song, L. Chirality 2013, 25,561. |
| [16] | (d) Huang,H. Y.; Zong, H.; Shen, B.; Yue,H. F.; Bian,G. L.; Song, L. Tetrahedron 2014, 70,1289. |
| [16] | (e) Yue,H. F.; Huang,H. Y.; Zong, H.; Bian,G. L.; Zong, H.; Li,F. L.; Song, L. Tetrahedron: Asymmetry 2014, 25,170. |
| [17] | Hatano, M.; Miyamoto, T.; Ishihara, K. Org. Lett. 2007, 9,4535. |
| [18] | Huang,H. Y.; Zong, H.; Bian,G. L.; Song, L. J. Org. Chem. 2015, 80,12614. |
| [19] | González-Martínez, D.; Gotor, V.; Gotor-Fernández, V. ChemCatChem 2019, 11,5800. |
| [20] | Brüning, F.; Nagae, H.; Käch, D.; Mashima,K. I.; Togni, A. Chem.-Eur. J. 2019, 25,10818. |
/
| 〈 |
|
〉 |