

铜催化的2H-1,2,3-三氮唑的绿色制备
收稿日期: 2021-01-06
修回日期: 2021-03-22
网络出版日期: 2021-04-16
基金资助
国家自然科学基金(21702051); 河南省博士后科学基金(001802033); 河南省科技攻关(212102311022); 河南师范大学青年科学基金(2016QK10)
Copper Catalyzed Synthesis of 2H-1,2,3-Triazoles in Green Solvent
Received date: 2021-01-06
Revised date: 2021-03-22
Online published: 2021-04-16
Supported by
National Natural Science Foundation of China(21702051); Postdoctoral Science Foundation of Henan Province(001802033); Henan Science and Technology Program(212102311022); Henan Normal University Science Foundation for Young Scholars(2016QK10)
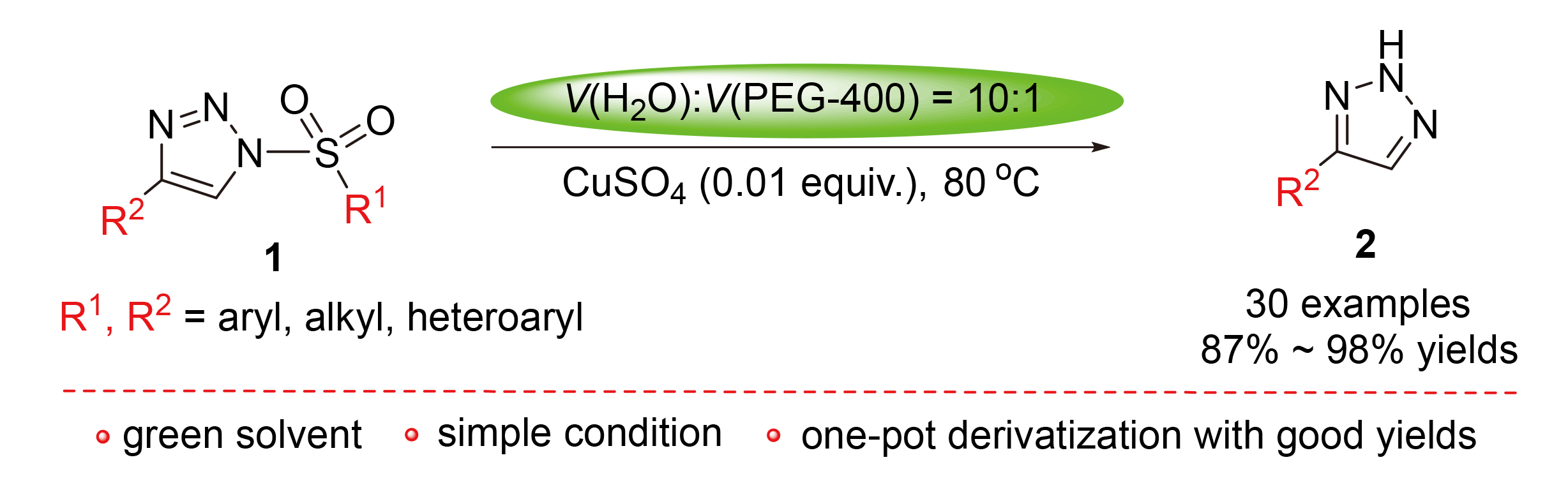
报道了4-取代-1-磺酰基-1,2,3-三氮唑在绿色溶剂中的催化水解反应, 以1 mol%硫酸铜作为催化剂, 在水/ PEG-400混合溶剂中以较高的收率高效合成了4-取代-2H-1,2,3-三氮唑. 该方法为制备2H-1,2,3-三氮唑类化合物提供了一种绿色、简单、有效的方法. 此外, 还用一锅法对2H-1,2,3-三氮唑进行了溴化、芳基化、烷基化的探索, 效果良好.
关键词: 2H-1,2,3-三氮唑; 硫酸铜; 三唑衍生物; 绿色溶剂
毕晶晶 , 孙潇潇 , 高松 , 陈长坡 , 张贵生 . 铜催化的2H-1,2,3-三氮唑的绿色制备[J]. 有机化学, 2021 , 41(7) : 2760 -2766 . DOI: 10.6023/cjoc202101010
The hydrolysis of 4-substituted 1-sulfonyl-1,2,3-triazoles induced by a catalytic amount of copper sulfate in the green solvent of H2O/PEG-400 was reported, providing a wide range of 4-substituted-2H-1,2,3-triazoles in excellent yields. The approach provides a green, simple and effective method for the preparation of 2H-1,2,3-triazole compounds. Bromination, arylation, and alkylation on 2H-1,2,3-triazoles by the one-pot method were also explored.

Key words: 2H-1,2,3-triazole; copper sulfate; triazole derivative; green solvent
| [1] | (a) Liu, Q.; Lü, Y.; Bao, P.; Yue, H.; Wei, W. Chin. J. Org. Chem. 2020, 40,4015 (in Chinese). |
| [1] | ( 刘启顺, 吕玉芬, 鲍鹏丽, 岳会兰, 魏伟, 有机化学, 2020, 40,4015.) |
| [1] | (b) Zhang, F.; Peng, X.; Ma, J. Chin. J. Org. Chem. 2019, 39,109 (in Chinese). |
| [1] | ( 张发光, 彭星, 马军安, 有机化学, 2019, 39,109.) |
| [1] | (c) Dong L.; Wang S.; Zhang X.; Cheng J.; Yuan Y. Chem. J. Chin. Univ. 2019, 40,927 (in Chinese). |
| [1] | ( 董丽蓉, 王思雨, 张小媚, 成佳佳, 袁耀锋, 高等学校化学学报, 2019, 40,927.) |
| [1] | (d) Liu, Z.; Hao, W.; Gao, W.; Zhu, G.; Li, X.; Tong, L.; Tang, B. Sci. China Chem. 2019, 62,1001. |
| [2] | (a) Yao, Y.; Ren, C.; Chen, L.; Zhong, L.; Xu, T.; Tan, C. Chin. J. Org. Chem. 2021, 41,2055 (in Chinese). |
| [2] | ( 姚阳意, 任朝丽, 陈丽, 钟良坤, 许天明, 谭成侠, 有机化学, 2021, 41,2055.) |
| [2] | (b) Sheng, C.; Zhang, W. Curr. Med. Chem. 2011; 18,733. |
| [2] | (c) Wang, C.; Zhou, F.; Zhou, J. Chin. J. Org. Chem. 2020, 40,3065 (in Chinese). |
| [2] | ( 王才, 周锋, 周剑, 有机化学, 2020, 40,3065.) |
| [2] | (d) Zheng, B.; Cheng, S.; Dong, H.; Zhu, J.; Han, Y.; Yang, L.; Hu, J. Acta Chim. Sinica 2020, 78,1089 (in Chinese). |
| [2] | ( 郑斌, 程盛, 董华泽, 朱金苗, 韩钰, 杨亮, 胡进明, 化学学报, 2020, 78,1089.) |
| [3] | (a) Zhang, L.; Zhao, J.; Wang, Y. Acta Chim. Sinica 2015, 73,1182 (in Chinese). |
| [3] | ( 张丽芳, 赵杰, 王勇, 化学学报, 2015, 73,1182.) |
| [3] | (b) Gao, C.; Chang, L.; Xu, Z.; Yan,X. -F.; Ding, C.; Zhao, F.; Wu, X.; Feng,L. -S. Eur. J. Med. Chem. 2019, 163,404. |
| [4] | (a) Bonandi, E.; Christodoulou,M. S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. Drug Discovery Today 2017, 22,1572. |
| [4] | (b) Cheng W.; Wang W.; Shang H.; Zhang H.; Guo Q.; Chen H.; Zou Z. J. China Pharm. Univ. 2018, 49,56 (in Chinese). |
| [4] | ( 成伟华, 王文倩, 尚海, 张宏武, 郭强, 陈虹, 邹忠梅, 中国药科大学学报, 2018, 49,56.) |
| [5] | Deng, L.; Cao, X.; Liu, Y.; Wan,J. -P. J. Org. Chem. 2019, 84,14179. |
| [6] | Zhang, W.; Kuang, C.; Yang, Q. Synthesis. 2010,283. |
| [7] | Barluenga, J.; Valdés, C.; Beltrán, G.; Escribano, M.; Aznar, F. Angew. Chem. Int. Ed. 2006, 45,6893. |
| [8] | Quan,X. -J.; Ren,Z. -H., Wang,Y. -Y.; Guan,Z. -H. Org. Lett. 2014, 16,5728. |
| [9] | Shu, W.; Zhang, X.; Zhang, X.; Li, M.; Wang, A.; Wu, A. J. Org. Chem. 2019, 84,14919. |
| [10] | Taylor,S. D.; Lohani,C. R. Org. Lett. 2016, 18,4412. |
| [11] | Gao, Y.; Lam, Y. Org. Lett. 2006, 8,3283. |
| [12] | Hu, Q.; Liu, Y.; Deng, X.; Li, Y.; Chen, Y. Adv. Synth. Catal. 2016, 358,1689. |
| [13] | Dong, H.; Zhang, D.; Fang, R.; Du, Q.; Dong, Zh.; Wei, H.; Shi, M.; Wang, F. Synth. Commun. 2018, 48,1227. |
| [14] | (a) Raushel, J.; Fokin,V. V. Org. Lett. 2010, 12,4952. |
| [14] | (b) Shao, C.; Wang, X.; Zhang, Q.; Luo, S.; Zhao, J.; Hu, Y. J. Org. Chem. 2011, 76,6832. |
| [14] | (c) Liu, Y.; Wang, X.; Xu, J.; Zhang, Q.; Zhao, Y.; Hu, Y. Tetrahedron 2011, 67,6294. |
| [15] | Wang, X.; Sidhu, K.; Zhang, L.; Campbell, S.; Haddad, N.; Reeves,D. C.; Krishnamurthy, D.; Senanayake,C. H. Org. Lett. 2009, 11,5490. |
| [16] | Liu, Y.; Yan, W.; Chen, Y.; Petersen,J. L.; Shi, X. Org. Lett. 2008, 10,5389. |
| [17] | (a) Zhang, M.; Zhang, A. J. Heterocycl. Chem. 2012, 49,721. |
| [17] | (b) Zhang, M.; Wang, Q.; Peng, Y.; Chen, Z.; Wan, C.; Chen, J.; Zhao, Y.; Zhang, R.; Zhang, A. Chem. Commun. 2019, 55,13048. |
| [18] | Deng, X.; Lei, X.; Nie, G.; Jia, L.; Li, Y.; Chen, Y. J. Org. Chem. 2017, 82,6163. |
/
| 〈 |
|
〉 |