

铜催化异氰酸酯加成反应机理研究
收稿日期: 2021-07-15
修回日期: 2021-08-15
网络出版日期: 2021-08-25
基金资助
国家自然科学基金(21903071); 河南省高校科技创新人才支持计划(20HASTIT004)
Mechanistic Study of Cu-Catalyzed Addition Reaction of lsocyanates
Received date: 2021-07-15
Revised date: 2021-08-15
Online published: 2021-08-25
Supported by
National Natural Science Foundation of China(21903071); Program for Science Technology Innovation Talents in Universities of Henan Province(20HASTIT004)
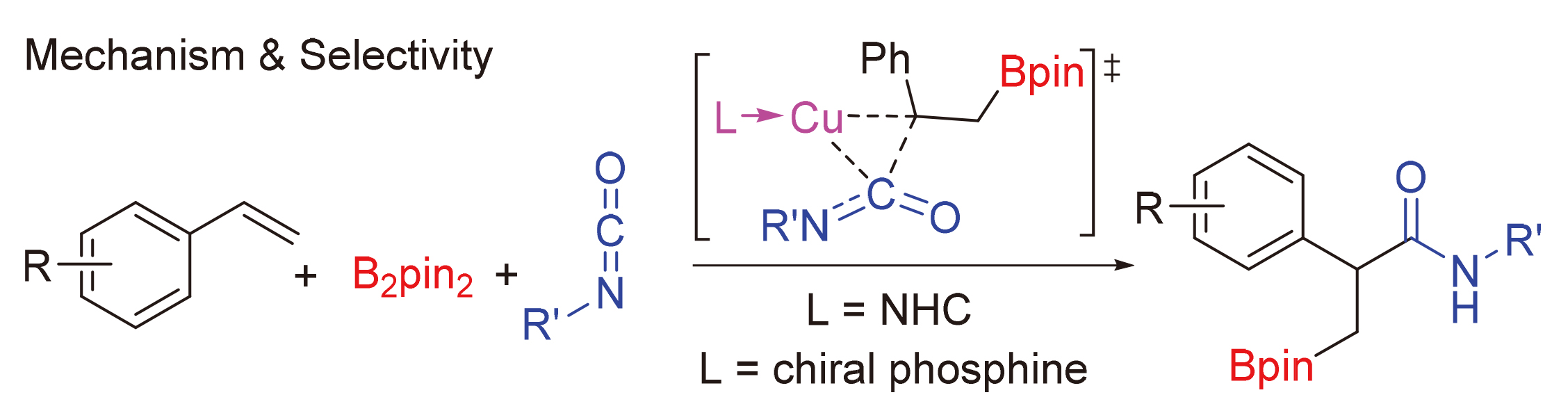
异氰酸酯作为一种重要的单碳碳源, 在合成化学中可以用于制备酰胺衍生物或杂环类化合物, 其活化和转化机制是一个重要的问题. 通过密度泛函理论(DFT)计算研究了亚铜催化异氰酸酯氢硼乙基化反应的机理. 研究结果表明, 该反应中叔丁醇亚铜是活性催化物种. 反应经历了叔丁醇亚铜与硼烷转金属化、烯烃插入、异氰酸酯插入、与叔丁醇锂转金属化再生叔丁醇亚铜等步骤. 其中, 异氰酸酯插入为决速步, 经历了一个特殊的三元环过渡态. 如果使用手性的亚膦酰胺作为配体, 则可实现立体选择性的异氰酸酯氢硼乙基化. 对映体选择性控制步为烯烃插入, 配体的空间调控决定了立体选择性. 反应决速步同样是异氰酸酯插入, 但相对使用卡宾配体, 该过程活化能较低. 因此, 亚膦酰胺-亚铜催化体系可能具有较高催化活性.
黄利 , 王毓浩 , 刘吉英 , 李世俊 , 张文静 , 蓝宇 . 铜催化异氰酸酯加成反应机理研究[J]. 有机化学, 2021 , 41(11) : 4347 -4352 . DOI: 10.6023/cjoc202107031
As a C1 synthetic block, isocyanates were widely used in the synthesis of amide derivatives or heterocyclic compounds. Density functional theory (DFT) calculation was employed to reveal the mechanism of Cu(I)-catalyzed hydroboraethylation of isocyanates. Cu(I)OtBu was considered as the active species in catalytic cycle. The catalytic cycle involves transmetallation with borane, alkene insertion, isocyanate insertion, and transmetallation with LiOtBu to yield lithium acetylamide product. The isocyanate insertion was considered as rate-determining step, which underwent a unique three- membered ring type transition state. The catalytic cycle with chiral phosphine ligand was also considered for this reaction. DFT calculation resulted that the enantioselectivity was determined at the alkene insertion step, which was controlled by steric effect of phosphine ligand. Moreover, it was also found that the activation free energy for the rate-determining step with phosphine ligand was lower than that with carbene ligand. Therefore, the phosphine ligand would lead to a higher reaction rate.

| [1] | Monie, F.; Grignard, B.; Thomassin, J.-M.; Mereau, R.; Tassaing, T.; Jerome, C.; Detrembleur, C. Angew. Chem., nt. Ed. 2020, 59, 17033. |
| [2] | Allen, M. A.; Ivanovich, R. A.; Beauchemin, A. M. Angew. Chem., nt. Ed. 2020, 59, 23188. |
| [3] | Sharpe, H. R.; Geer, A. M.; Lewis, W.; Blake, A. J.; Kays, D. L. Angew. Chem.,Int. Ed. 2017, 56, 4845. |
| [4] | Zheng, S.; Primer, D. N.; Molander, G. A. ACS Catal. 2017, 7, 7957. |
| [5] | Mohjer, F.; Mofatehnia, P.; Rangraz, Y.; Heravi, M. M. J. Organomet. Chem. 2021, 936, 121712. |
| [6] | Cui, C.-X.; Chen, H.; Li, S.-J.; Zhang, T.; Qu, L.-B.; Lan, Y. Coord. Chem. Rev. 2020, 412, 213251. |
| [7] | Zhang, C.; Zhu, Y. Y.; Wei, D. H.; Sun, D. Z.; Zhang, W. J.; Tang, M. S. J. Phys. Chem. A 2010, 114, 2913. |
| [8] | Khan, A.; Xing, J. X.; Zhao, J. M.; Kan, Y. H.; Zhang, W. B.; Zhang, Y. J. Chem.-Eur. J. 2015, 21, 120. |
| [9] | Yingcharoen, P.; Natongchai, W.; Poater, A.; D'Elia, V. Catal. Sci. Technol. 2020, 10, 5544. |
| [10] | Bruffaerts, J.; von Wolff, N.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. J. Am. Chem. Soc. 2019, 141, 16486. |
| [11] | Yang, Y.; Anker, M. D.; Fang, J.; Mahon, M. F.; Maron, L.; Weetman, C.; Hill, M. S. Chem. Sci. 2017, 8, 3529. |
| [12] | Lu, C. R.; Hu, L. J.; Zhao, B.; Yao, Y. M. Organometallics 2019, 38, 2167. |
| [13] | Yang, Y.; Canty, A. J.; O'Hair, R. A. J. J. Mass Spectrom. 2021, 56, e4579. |
| [14] | Alves, L. G.; Madeira, F.; Munhá, R. F.; Barros, S.; Veiros, L. F.; Martins, A. M. Dalton Trans. 2015, 44, 1441. |
| [15] | Fiorito, D.; Liu, Y.; Besnard, C.; Mazet, C. J. Am. Chem. Soc. 2020, 142, 623. |
| [16] | Peris, E. Chem. Rev. 2018, 118, 9988. |
| [17] | Kuwata, S.; Hahn, F. E. Chem. Rev. 2018, 118, 9642. |
| [18] | Xu, G. Q.; Senanayake, C. H.; Tang, W. J. Acc. Chem. Res. 2019, 52, 1101. |
| [19] | Fu, W. Z.; Tang, W. J. ACS Catal. 2016, 6, 4814. |
| [20] | de Figueiredo, R. M.; Suppo, J.-S.; Campagne, J.-M. Chem. Rev. 2016, 116, 12029. |
| [21] | (a) Kohn, W.; Sham, L. J. Phys. Rev. 1965, 140, A1133. |
| [21] | (b) Kohn, W. Rev. Mod. Phys. 1999, 71, 1253. |
| [22] | Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, N. J.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J.Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013. |
| [23] | (a) Becke, A. D. J. Chem. Phys. 1993, 98, 5648. |
| [23] | (b) Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623. |
| [23] | (c) Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785. |
| [24] | (a) Dolg, M.; Stoll, H.; Preuss, H. J. Chem. Phys. 1989, 90, 1730. |
| [24] | (b) Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. J. Chem. Phys. 1987, 86, 866. |
| [25] | (a) Francl, M. M. J. Chem. Phys. 1982, 77, 3654. |
| [25] | (b) Hehre, W. J. J. Chem. Phys. 1972, 56, 2257. |
| [25] | (c) Hariharan, P. C.; Pople, J. A. Theor. Chim. Acta 1973, 28, 213. |
| [26] | (a) Sang-Aroon, W.; Ruangpornvisuti, V. Int. J. Quantum Chem. 2008, 108, 1181. |
| [26] | (b) Tomasi, J.; Mennucci, B.; Cancès, E. J. Mol. Struct. 1999, 464, 211. |
| [27] | (a) Gonzalez, C.; Schlegel, H. B. J. Chem. Phys. 1989, 90, 2154. |
| [27] | (b) Gonzalez, C.; Schlegel, H. B. J. Phys. Chem. 1990, 94, 5523. |
| [28] | Zhao, Y.; Truhlar, D. Theor. Chem. Acc. 2008, 120, 215. |
| [29] | (a) Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297. |
| [29] | (b) Cancès, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032. |
| [30] | (a) Liu, C.; Li, S.-J.; Han, P.; Qu, L.-B.; Lan, Y. Mol. Catal. 2021, 499, 111318. |
| [30] | (b) Shen, B.; Liu, S.; Zhu, L.; Zhong, K.; Liu, F.; Chen, H.; Bai, R.; Lan, Y. Organometallics, 2020, 39, 2813. |
| [30] | (c) Wang, Y.; Liao, W.; Huang, G.; Xia, Y.; Yu, Z.-X. J. Org. Chem. 2014, 79, 5684. |
/
| 〈 |
|
〉 |