

钌选择性催化烯丙醇无受体脱氢合成α,β-不饱和羰基化合物
收稿日期: 2021-07-16
修回日期: 2021-08-15
网络出版日期: 2021-08-25
基金资助
国家自然科学基金(21973113); 国家自然科学基金(21977019); 广东省自然科学杰出青年基金(2015A030306027)
Ruthenium Catalyzed Selective Acceptorless Dehydrogenation of Allylic Alcohols to α,β-Unsaturated Carbonyls
Received date: 2021-07-16
Revised date: 2021-08-15
Online published: 2021-08-25
Supported by
National Natural Science Foundation of China(21973113); National Natural Science Foundation of China(21977019); Guangdong Natural Science Funds for Distinguished Young Scholar(2015A030306027)
过渡金属催化烯丙醇的选择性脱氢氧化以得到相应的α,β-不饱和羰基化合物在最近广受关注, 但是很多方法需要用到化学计量的氧化剂, 这会带来大量的废弃副产物, 原子经济性不足. 本工作开发了一种简单易得的[RuCl2(p- cymene)]2催化系统, 可用于高效地催化烯丙醇选择性脱氢合成α,β-不饱和羰基化合物, 而无需使用额外的氧化剂或H2受体.
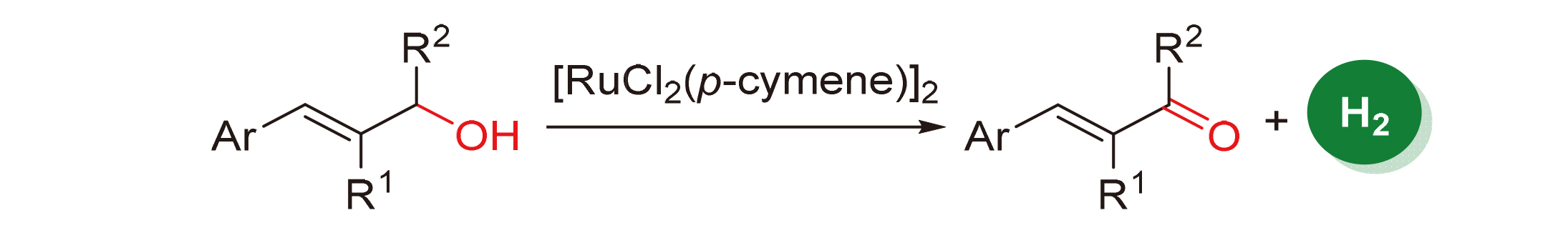
关键词: 钌; 无受体脱氢; 烯丙醇; α,β-不饱和羰基化合物
刘嘉豪 , 张世冬 , 栾自鸿 , 刘艳 , 柯卓锋 . 钌选择性催化烯丙醇无受体脱氢合成α,β-不饱和羰基化合物[J]. 有机化学, 2021 , 41(11) : 4361 -4369 . DOI: 10.6023/cjoc202107037
Transition metal-catalyzed allyllic alcohol selective dehydrogenation to generate the corresponding α,β-unsaturated carbonyl compound has attached great attention. However, most of these methods require stoichiometric quantities of oxidants, which will bring a lot of by-products and lack of atomic economy. Herein, a simple [RuCl2(p-cymene)]2 catalyzed system to efficiently catalyze the selective dehydrogenation of allyl alcohol to form α,β-unsaturated carbonyls without the use of additional oxidants or H2 acceptors has been developed.
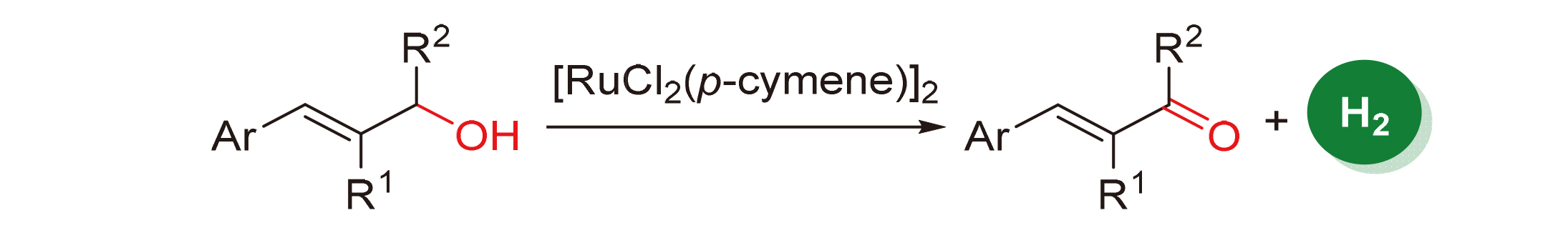
| [1] | (a) Dobereiner, G. E.; Crabtree, R. H. Chem. Rev. 2010, 110, 681. |
| [1] | (b) Choi, J.; MacArthur, A. H. R.; Brookhart, M.; Goldman, A. S. Chem. Rev. 2011, 111, 1761. |
| [1] | (c) Kumar, A.; Bhatti, T. M.; Goldman, A. S. Chem. Rev. 2017, 117, 12357. |
| [2] | (a) van der Drift, R. C.; Bouwman, E.; Drent, E. J. Organomet. Chem. 2002, 650, 1. |
| [2] | (b) Uma, R.; Crévisy, C.; Grée, R. Chem. Rev. 2003, 103, 27. |
| [2] | (c) Cadierno, V.; Crochet, P.; Gimeno, J. Synlett 2008, 1105. |
| [2] | (d) Mantilli, L.; Mazet, C. Chem. Lett. 2011, 40, 341. |
| [2] | (e) Ahlsten, N.; Bartoszewicz, A.; Martín-Matute, B. Dalton Trans. 2012, 41, 1660. |
| [3] | (a) Nakano, T.; Ishii, Y.; Ogawa, M. J. Org. Chem. 1987, 52, 4855. |
| [3] | (b) Adam, W.; Gelalcha, F. G.; Saha-Möller, C. R.; Stegmann, V. R. J. Org. Chem. 2000, 65, 1915. |
| [3] | (c) Kakiuchi, N.; Maeda, Y.; Nishimura, T.; Uemura, S. J. Org. Chem. 2001, 66, 6620. |
| [3] | (d) Johnston, E. V.; Verho, O.; Kärkäs, M. D.; Shakeri, M.; Tai, C.-W.; Palmgren, P.; Eriksson, K.; Oscarsson, S.; Bäckvall, J.-E. Chem.-Eur. J. 2012, 18, 12202. |
| [3] | (e) Hill-Cousins, J. T.; Kuleshova, J.; Green, R. A.; Birkin, P. R.; Pletcher, D.; Underwood, T. J.; Leach, S. G.; Brown, R. C. D. ChemSusChem 2012, 5, 326. |
| [3] | (f) Nishii, T.; Ouchi, T.; Matsuda, A.; Matsubara, Y.; Haraguchi, Y.; Kawano, T.; Kaku, H.; Horikawa, M.; Tsunoda, T. Tetrahedron Lett. 2012, 53, 5880. |
| [3] | (g) Xing, Y.; Li, C.; Meng, J.; Zhang, Z.; Wang, X.; Wang, Z.; Ye, Y.; Sun, K. Adv. Synth. Catal. 2021, 363, 3913. |
| [3] | (h) Mancuso, A. J.; Swern, D. Synthesis 1981, 1981, 165. |
| [4] | (a) Wang, G. Z.; Bäckvall, J.-E. J. Chem. Soc., Chem. Commun. 1992, 337. |
| [4] | (b) Almeida, M. L. S.; Beller, M.; Wang, G.-Z.; Bäckvall, J.-E.; Chem.-Eur. J. 1996, 2, 1533. |
| [4] | (c) Almeida, M. L. S.; Kočovský, P.; Bäckvall, J.-E. J. Org. Chem. 1996, 61, 6587. |
| [4] | (d) Nishibayashi, Y.; Yamauchi, A.; Onodera, G.; Uemura, S. J. Org. Chem. 2003, 68, 5875. |
| [4] | (e) Gauthier, S.; Scopelliti, R.; Severin, K. Organometallics 2004, 23, 3769. |
| [4] | (f) Yi, C. S.; Zeczycki, T. N.; Guzei, I. A. Organometallics 2006, 25, 1047. |
| [4] | (g) Nielsen, M.; Kammer, A.; Cozzula, D.; Junge, H.; Gladiali, S.; Beller, M. Angew. Chem., Int. Ed. 2011, 50, 9593. |
| [4] | (h) Chelucci, G.; Baldino, S.; Baratta, W. Coord. Chem. Rev. 2015, 300, 29. |
| [4] | (i) Zhang, W.; Meng, C.; Liu, Y.; Tang, Y.; Li, F. Adv. Synth. Catal. 2018, 360, 3751. |
| [4] | (j) Udvardy, A.; Joó, F.; Kathó, Á. Coord. Chem. Rev. 2021, 438, 213871. |
| [4] | (k) Zeng, M.; Song, C.; Cui, D. Chin. J. Org. Chem. 2017, 37, 1352. (in Chinese) |
| [4] | (曾明, 宋婵, 崔冬梅, 有机化学, 2017, 37, 1352.) |
| [5] | (a) Coleman, M. G.; Brown, A. N.; Bolton, B. A.; Guan, H. Adv. Synth. Catal. 2010, 352, 967. |
| [5] | (b) Chakraborty, S.; Lagaditis, P. O.; Förster, M.; Bielinski, E. A.; Hazari, N.; Holthausen, M. C.; Jones, W. D.; Schneider, S. ACS Catal. 2014, 4, 3994. |
| [5] | (c) Budweg, S.; Wei, Z.; Jiao, H.; Junge, K.; Beller, M. ChemSusChem 2019, 12, 2988. |
| [5] | (d) Chun, S.; Ahn, J.; Putta, R. R.; Lee, S. B.; Oh, D.-C.; Hong, S. J. Org. Chem. 2020, 85, 15314. |
| [5] | (e) Budweg, S.; Junge, K.; Beller, M. Catal. Sci. Technol. 2020, 10, 3825. |
| [6] | (a) Suzuki, T.; Morita, K.; Tsuchida, M.; Hiroi, K. J. Org. Chem. 2003, 68, 1601. |
| [6] | (b) Hanasaka, F.; Fujita, K.; Yamaguchi, R. Organometallics 2004, 23, 1490. |
| [6] | (c) Hanasaka, F.; Fujita, K.; Yamaguchi, R. Organometallics 2005, 24, 3422. |
| [6] | (d) Hanasaka, F.; Fujita, K.; Yamaguchi, R. Organometallics 2006, 25, 4643. |
| [6] | (e) Fujita, K.; Yoshida, T.; Imori, Y.; Yamaguchi, R. Org. Lett. 2011, 13, 2278. |
| [6] | (f) Musa, S.; Shaposhnikov, I.; Cohen, S.; Gelman, D. Angew. Chem., Int. Ed. 2011, 50, 3533. |
| [6] | (g) Polukeev, A. V.; Petrovskii, P. V.; Peregudov, A. S.; Ezernitskaya, M. G.; Koridze, A. A. Organometallics 2013, 32, 1000. |
| [6] | (h) Shi, Y.; Suguri, T.; Kojima, S.; Yamamoto, Y. J. Organomet. Chem. 2015, 799-800, 7. |
| [6] | (i) Polukeev, A. V.; Wendt, O. F. Organometallics 2017, 36, 639. |
| [7] | (a) Join, B.; Möller, K.; Ziebart, C.; Schröder, K.; Gördes, D.; Thurow, K.; Spannenberg, A.; Junge, K.; Beller, M. Adv. Synth. Catal. 2011, 353, 3023. |
| [7] | (b) Könning, D.; Olbrisch, T.; Sypaseuth, F. D.; Tzschucke, C. C.; Christmann, M. Chem. Commun. 2014, 50, 5014. |
| [8] | (a) Oppenauer, R. V. Recl. Trav. Chim. Pays-Bas 1937, 56, 137. |
| [8] | (b) de Graauw, C. F.; Peters, J. A.; van Bekkum, H.; Huskens, J. Synthesis 1994, 1007. |
| [8] | (c) Gunanathan, C.; Milstein, D. Science 2013, 341, 1229712. |
| [8] | (d) Song, H.; Kang, B.; Hong, S. H. ACS Catal. 2014, 4, 2889. |
| [8] | (e) Huang, F.; Liu, Z.; Yu, Z. Angew. Chem., Int. Ed. 2016, 55, 862. |
| [8] | (f) Kallmeier, F.; Kempe, R. Angew. Chem., Int. Ed. 2018, 57, 46. |
| [8] | (g) Huang, M.; Liu, J.; Li, Y.; Lan, X.-B.; Su, P.; Zhao, C.; Ke, Z. Catal. Today 2020. |
| [9] | (a) Dobson, A.; Robinson, S. D. Inorg. Chem. 1977, 16, 137. |
| [9] | (b) Hamid, M. H. S. A.; Slatford, P. A.; Williams, J. M. J. Adv. Synth. Catal. 2007, 349, 1555. |
| [9] | (c) Watson, A. J. A.; Williams, J. M. J. Science 2010, 329, 635. |
| [9] | (d) Guillena, G.; Ramon, D. J.; Yus, M. Chem. Rev. 2010, 110, 1611. |
| [9] | (e) Johnson, T. C.; Morris, D. J.; Wills, M. Chem. Soc. Rev. 2010, 39, 81. |
| [9] | (f) Trincado, M.; Banerjee, D.; Grützmacher, H. Energy Environ. Sci. 2014, 7, 2464. |
| [9] | (g) Kim, S. W.; Zhang, W.; Krische, M. J. Acc. Chem. Res. 2017, 50, 2371. |
| [9] | (h) Chelucci, G. Coord. Chem. Rev. 2017, 331, 1. |
| [9] | (i) Crabtree, R. H. Chem. Rev. 2017, 117, 9228. |
| [9] | (j) Corma, A.; Navas, J.; Sabater, M. J. Chem. Rev. 2018, 118, 1410. |
| [9] | (k) Hu, B.; Zhang, Y.; Yin, G.; Chen, D. Chin. J. Org. Chem. 2020, 40, 53. (in Chinese) |
| [9] | (胡博文, 张宇哲, 尹鸽平, 陈大发, 有机化学, 2020, 40, 53.) |
| [9] | (l) Wang, C.; Xiao, J. Chin. J. Org. Chem. 2020, 40, 2182. (in Chinese) |
| [9] | (王超, 肖建良, 有机化学, 2020, 40, 2182.) |
| [9] | (m) Hao, Z.; Liu, K.; Feng, Q.; Dong, Q.; Ma, D.; Han, Z.; Lu, G.-L.; Lin, J. Chin. J. Chem. 2021, 39, 121. |
| [10] | (a) Choi, J. H.; Kim, N.; Shin, Y. J.; Park, J. H.; Park, J. Tetrahedron Lett. 2004, 45, 4607. |
| [10] | (b) Kim, W.-H.; Park, I. S.; Park, J. Org. Lett. 2006, 8, 2543. |
| [10] | (c) Mitsudome, T.; Mikami, Y.; Funai, H.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Angew. Chem., Int. Ed. 2008, 47, 138. |
| [10] | (d) Mitsudome, T.; Mikami, Y.; Ebata, K.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Chem. Commun. 2008, 4804. |
| [10] | (e) Shimizu, K.; Sugino, K.; Sawabe, K.; Satsuma, A. Chem.-Eur. J. 2009, 15, 2341. |
| [10] | (f) Fang, W.; Chen, J.; Zhang, Q.; Deng, W.; Wang, Y. Chem.-Eur. J. 2011, 17, 1247. |
| [10] | (g) Marella, R. K.; Prasad Neeli, C. K.; Rao Kamaraju, S. R.; Burri, D. R. Catal. Sci. Technol. 2012, 2, 1833. |
| [10] | (h) Shimizu, K.; Kon, K.; Seto, M.; Shimura, K.; Yamazaki, H.; Kondo, J. N. Green Chem. 2013, 15, 418. |
| [10] | (i) Shimizu, K.; Kon, K.; Shimura, K.; Hakim, S. S. M. A. J. Catal. 2013, 300, 242. |
| [10] | (j) Kon, K.; Hakim Siddiki, S. M. A.; Shimizu, K. J. Catal. 2013, 304, 63. |
| [10] | (k) Yi, J.; Miller, J. T.; Zemlyanov, D. Y.; Zhang, R.; Dietrich, P. J.; Ribeiro, F. H.; Suslov, S.; Abu-Omar, M. M. Angew. Chem., Int. Ed. 2014, 53, 833. |
| [10] | (l) Chen, J.; Fang, W.; Zhang, Q.; Deng, W.; Wang, Y. Chem. Asian J. 2014, 9, 2187. |
| [10] | (m) González Miera, G.; Martínez-Castro, E.; Martín-Matute, B. Organometallics 2018, 37, 636. |
| [11] | Ren, K.; Hu, B.; Zhao, M.; Tu, Y.; Xie, X.; Zhang, Z. J. Org. Chem. 2014, 79, 2170. |
| [12] | (a) Tseng, K.-N. T.; Kampf, J. W.; Szymczak, N. K. ACS Catal. 2015, 5, 5468. |
| [12] | (b) Hou, C.; Zhang, Z.; Zhao, C.; Ke, Z. Inorg. Chem. 2016, 55, 6539. |
| [12] | (c) Wang, Q.; Chai, H.; Yu, Z. Organometallics 2017, 36, 3638. |
| [13] | (a) Soai, K.; Yokoyama, S.; Mochida, K. Synthesis 1987, 1987, 647. |
| [13] | (b) Håkansson, A. E.; Palmelund, A.; Holm, H.; Madsen, R. Chem.- Eur. J. 2006, 12, 3243. |
| [14] | Zhu, Y.; Colomer, I.; Donohoe, T. J. Chem. Commun. 2019, 55, 10316. |
| [15] | Krätzschmar, F.; Kaßel, M.; Delony, D.; Breder, A. Chem.-Eur. J. 2015, 21, 7030. |
| [16] | Papa Spadafora, B.; Moreira Ribeiro, F. W.; Matsushima, J. E.; Ariga, E. M.; Omari, I.; Soares, P. M. A.; de Oliveira-Silva, D.; Vinhato, E.; McIndoe, J. S.; Carita Correra, T.; Rodrigues, A. Org. Biomol. Chem. 2021, 19, 5595. |
| [17] | Hu, D. X.; Shibuya, G. M.; Burns, N. Z. J. Am. Chem. Soc. 2013, 135, 12960. |
| [18] | Talwar, D.; Wu, X.; Saidi, O.; Salguero, N. P.; Xiao, J. Chem.-Eur. J. 2014, 20, 12835. |
| [19] | Cignarella, G.; Occelli, E.; Testa, E. J. Med. Chem. 1965, 8, 326. |
| [20] | Tomita, R.; Mantani, K.; Hamasaki, A.; Ishida, T.; Tokunaga, M. Chem.-Eur. J. 2014, 20, 9914. |
| [21] | Balcells, S.; Haughey, M. B.; Walker, J. C. L.; Josa-Culleré, L.; Towers, C.; Donohoe, T. J. Org. Lett. 2018, 20, 3583. |
| [22] | Li, H.; Chen, H.; Zhou, Y.; Huang, J.; Yi, J.; Zhao, H.; Wang, W.; Jing, L. Chem. Asian J. 2020, 15, 555. |
| [23] | Wang, R.; Tang, Y.; Xu, M.; Meng, C.; Li, F. J. Org. Chem. 2018, 83, 2274. |
| [24] | Li, C.; Chen, H.; Li, J.; Li, M.; Liao, J.; Wu, W.; Jiang, H. Adv. Synth. Catal. 2018, 360, 1600. |
| [25] | Meiß, R.; Kumar, K.; Waldmann, H. Chem.-Eur. J. 2015, 21, 13526. |
| [26] | Yang, Y.; Jiang, J.; Qimei, L.; Yan, X.; Zhao, J.; Yuan, H.; Qin, Z.; Wang, M. Molecules 2010, 15, 7075. |
| [27] | Morrill, C.; Grubbs, R. H. J. Am. Chem. Soc. 2005, 127, 2842. |
| [28] | Kim, D. E.; Kwak, J.; Kim, I. S.; Jeong, N. Adv. Synth. Catal. 2009, 351, 97. |
| [29] | Liu, J.; Zhu, J.; Jiang, H.; Wang, W.; Li, J. Chem. Commun. 2010, 46, 415. |
| [30] | Susanto, W.; Chu, C.-Y.; Ang, W. J.; Chou, T.-C.; Lo, L.-C.; Lam, Y. J. Org. Chem. 2012, 77, 2729. |
| [31] | Zhang, Z.; Wang, Q.; Chen, C.; Han, Z.; Dong, X.-Q.; Zhang, X. Org. Lett. 2016, 18, 3290. |
| [32] | Chen, X.; Zhang, Y.; Wan, H.; Wang, W.; Zhang, S. Chem. Commun. 2016, 52, 3532. |
| [33] | Ahmed, M.; Brand, H. E. A.; Peterson, V. K.; Clegg, J. K.; Kepert, C. J.; Price, J. R.; Powell, B. J.; Neville, S. M. Dalton Trans. 2021, 50, 1434. |
| [34] | Chavhan, S. W.; Cook, M. J. Chem.-Eur. J. 2014, 20, 4891. |
| [35] | Zhang, X.-W.; Jiang, G.-Q.; Lei, S.-H.; Shan, X.-H.; Qu, J.-P.; Kang, Y.-B. Org. Lett. 2021, 23, 1611. |
| [36] | An, X.-L.; Chen, J.-R.; Li, C.-F.; Zhang, F.-G.; Zou, Y.-Q.; Guo, Y.-C.; Xiao, W.-J. Chem. Asian J. 2010, 5, 2258. |
| [37] | Guo, S.-H.; Xing, S.-Z.; Mao, S.; Gao, Y.-R.; Chen, W.-L.; Wang, Y.-Q. Tetrahedron Lett. 2014, 55, 6718. |
| [38] | Zhang, G.; Han, X.; Luan, Y.; Wang, Y.; Wen, X.; Ding, C. Chem. Commun. 2013, 49, 7908. |
| [39] | You, S.; Zhang, R.; Cai, M. Synthesis 2021, 53, 1962. |
| [40] | Yu, C.-W.; Chen, G. S.; Huang, C.-W.; Chern, J.-W. Org. Lett. 2012, 14, 3688. |
| [41] | Tigineh, G. T.; Liu, L.-K. J. Chin. Chem. Soc. 2019, 66, 1729. |
| [42] | Yu, B.; Zhao, Y.; Zhang, H.; Xu, J.; Hao, L.; Gao, X.; Liu, Z. Chem. Commun. 2014, 50, 2330. |
| [43] | Chen, X.-Y.; Sorensen, E. J. J. Am. Chem. Soc. 2018, 140, 2789. |
| [44] | Shen, D.; Miao, C.; Wang, S.; Xia, C.; Sun, W. Org. Lett. 2014, 16, 1108. |
| [45] | Konishi, H.; Kumon, M.; Yamaguchi, M.; Manabe, K. Tetrahedron 2020, 76, 131639. |
| [46] | Troxler, F.; Harnisch, A.; Bormann, G.; Seemann, F.; Szabo, L. Helv. Chim. Acta 1968, 51, 1616. |
/
| 〈 |
|
〉 |