

基于“一石二鸟”策略的羧基无痕导向其邻位C—H键官能团化反应
收稿日期: 2021-06-11
修回日期: 2021-08-11
网络出版日期: 2021-09-08
基金资助
国家自然科学基金(21761021); 国家自然科学基金(21861026)
ortho-C—H Bond Functionalization of Carboxylic Acid Using Carboxyl as a Traceless Directing Group Based on the Strategy of “Two Birds with One Stone”
Received date: 2021-06-11
Revised date: 2021-08-11
Online published: 2021-09-08
Supported by
National Natural Science Foundation of China(21761021); National Natural Science Foundation of China(21861026)
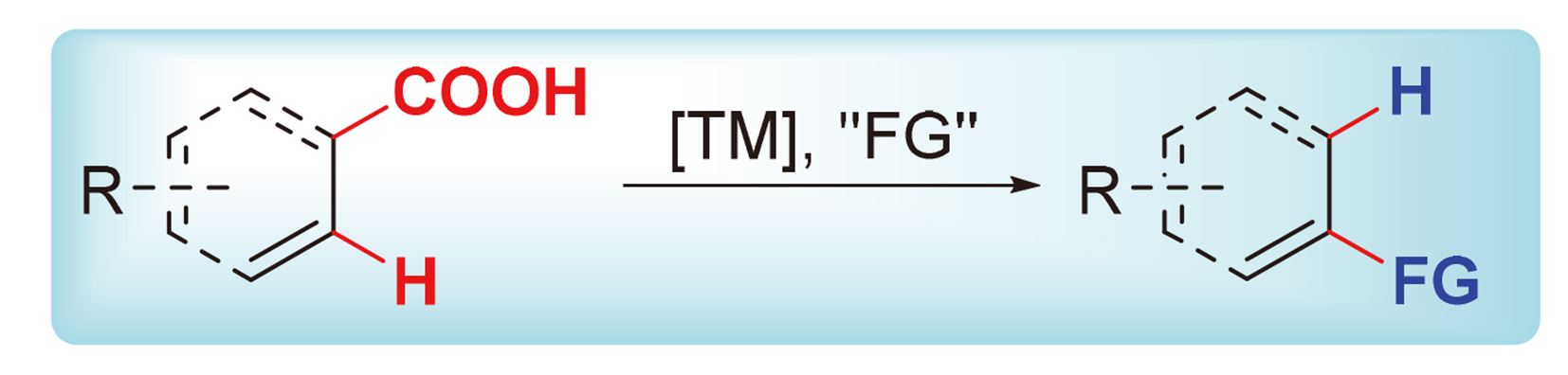
C—H键作为有机化合物的基本单元, 实现其直接的C—H键官能团化反应是简洁的合成方法. 羧酸广泛存在于自然界, 基于羧基的导向基团与离去基团双重角色所驱动的过渡金属催化羧酸邻位C—H键官能团化可控合成, 不仅规避了C—H键活化过程中导向基团的额外引入与移除, 也彰显了基于羧基“一石二鸟”策略的C—H键活化简洁性与脱羧绿色性. 因此, 基于“一石二鸟”策略的羧基无痕导向其邻位C—H键官能团化反应, 能为可控定向合成提供新的策略和方法, 在合成化学上具有显著意义. 根据参与反应的偶联底物类型, 分别介绍了基于“一石二鸟”策略的过渡金属催化羧酸邻位C—H键活化与含重键试剂、芳基化试剂及含杂原子试剂的反应, 并对相关的一些反应机理进行了探讨.
付拯江 , 曹晰晗 , 尹健 , 苟振宇 , 伊学政 , 蔡琥 . 基于“一石二鸟”策略的羧基无痕导向其邻位C—H键官能团化反应[J]. 有机化学, 2022 , 42(1) : 67 -74 . DOI: 10.6023/cjoc202106024
Since C—H bond is the basic unit of organic compounds, it’s considered to be a concise synthesis method to implement direct functionalization of C—H bond. As carboxylic acids are widely found in nature, carboxyl group can act as both directing group and leaving group to drive transition metal-catalyzed controllable transformation of ortho-C—H bond functionalization of carboxylic acid that not only avoids the extra introduction and removal of the directing group in the process of C—H bond activation, but also highlights the simplicity of C—H bond activation and green of decarboxylation with “one stone and two birds” strategy of the carboxyl group. Therefore, basing on the strategy of “two birds with one stone”, ortho-C—H bond functionalization of carboxylic acid using carboxyl as a traceless directing group can provide a new strategy and method for controllable and directional synthesis, which has considerable significance in synthetic chemistry. According to the types of coupling partners involved in the transformations, transition metal-catalyzed ortho-C—H bond activations of carboxylic acids with multiple-bond-containing reagents, arylating compounds and heteroatom-containing chemicals are reviewed respectively based on the strategy of “two birds with one stone”, and the relevant reaction mechanisms are discussed.

| [1] | Murai, S.; Kakiuchi, F.; Sekine, S.; Tanaka, Y.; Kanatani, A.; Sonoda, M.; Chatani, N. Nature 1993, 366, 529. |
| [2] | Zhang, F.; Spring, D. R. Chem. Soc. Rev. 2014, 43, 6906. |
| [3] | Font, M.; Quibell, J. M.; Perry, G. J. P.; Larrosa, I. Chem. Commun. 2017, 53, 5584. |
| [4] | Samanta, R.; Antonchick, A. P. Angew. Chem., Int. Ed. 2011, 50, 5217. |
| [5] | Xu, Y.; Yang, X.; Zhou, X.; Kong, L.; Li, X. Org. Lett. 2017, 19, 4307. |
| [6] | Kumar, G. S.; Kumar, P.; Kapur, M. Org. Lett. 2017, 19, 2494. |
| [7] | (a) Kuai, C.; Wang, L.; Li, B.; Yang, Z.; Cui, X. Org. Lett. 2017, 19, 2102. |
| [7] | (b) Ling, F.; Ai, C.; Lv, Y.; Zhong, W. Adv. Synth. Catal. 2017, 359, 3707. |
| [8] | Wang, J.; Wang, M.; Chen, K.; Zha, S.; Song, C.; Zhu, J. Org. Lett. 2016, 18, 1178. |
| [9] | (a) Barsu, N.; Sen, M.; Premkumar, J. R.; Sundararaju, B. Chem. Commun. 2016, 52, 1338. |
| [9] | (b) Huang, X.; Huang, J.; Du, C.; Zhang, X.; Song, F.; You, J. Angew. Chem., Int. Ed. 2013, 52, 12970. |
| [10] | Wang, J.; Wang, M.; Chen, K.; Zha, S.; Song, C.; Zhu, J. Org. Lett. 2016, 18, 1178. |
| [11] | Wu, X.-L.; Dong, L. Org. Lett. 2018, 20, 6990. |
| [12] | Wu, Z.; Ma, D.; Zhou, B.; Ji, X.; Ma, X.; Wang, X.; Zhang, Y. Angew. Chem., Int. Ed. 2017, 56, 12288. |
| [13] | Zheng, L.; Hua, R. Chem.-Eur. J. 2014, 20, 2352. |
| [14] | Cornella, J.; Larrosa, I. Synthesis 2012, 44, 653. |
| [15] | (a) Luo, F. Chin. J. Org. Chem. 2019, 39, 3084. (in Chinese) |
| [15] | 罗飞华, 有机化学, 2019, 39, 3084). |
| [15] | (b) Font, M.; Quibell, J. M.; Perry, G. J. P.; Larrosa, I. Chem. Commun. 2017, 53, 5584. |
| [16] | (a) Zhang, T.; Wang, N.-X.; Xing, Y. J. Org. Chem. 2018, 83, 7559. |
| [16] | (b) Wei, Y.; Hu, P.; Zhang, M.; Su, W. Chem. Rev. 2017, 117, 8864. |
| [16] | (c) Chen, Y.; Tian, S.-K. Chin. J. Chem. 2013, 31, 37. |
| [16] | (d) Guan, B.; Xu, X.; Wang, H.; Li, X. Chin. J. Org. Chem. 2016, 36, 1564. (in Chinese) |
| [16] | (关保川, 许孝良, 王红, 李小年, 有机化学, 2016, 36, 1564.) |
| [16] | (e) Yin, X.; Li, W.; Zhao, B.; Cheng, K. Chin. J. Org. Chem. 2018, 38, 2879. (in Chinese) |
| [16] | (殷晓婷, 李文炅, 赵保丽, 程凯, 有机化学, 2018, 38, 2879.) |
| [16] | (f) Fu, Z.; Li, Z.; Xiong, Q.; Cai, H. Chin. J. Org. Chem. 2015, 35, 984. (in Chinese) |
| [16] | (付拯江, 李兆杰, 熊起恒, 蔡琥, 有机化学, 2015, 35, 984.) |
| [17] | (a) Trita, A.; Biafora, A.; Drapeau, M.; Weber, P.; Goossen, L. Angew. Chem., Int. Ed. 2018, 57, 14580. |
| [17] | (b) Dana, S.; Mandal, A.; Sahoo, H.; Mallik, S.; Grandhi, G.; Baidya, M. Org. Lett. 2018, 20, 716. |
| [17] | (c) Han, W.; Pu, F.; Li, C.; Liu, Z.; Fan, J.; Shi, X. Adv. Synth. Catal. 2018, 360, 1358. |
| [18] | (a) Tan, G.; You, Q.; Lan, J.; You, J. Angew. Chem., Int. Ed. 2018, 57, 6309. |
| [18] | (b) Wu, Z.; Chen, S.; Hu, C.; Li, Z.; Xiang, H.; Zhou, X. ChemCatChem 2013, 5, 2839. |
| [18] | (c) Chiong, H.; Pham, Q.; Daugulis, O. J. Am. Chem. Soc. 2007, 129, 9879. |
| [19] | Cheng, G.; Li, T.; Yu, J. J. Am. Chem. Soc. 2015, 137, 10950. |
| [20] | Mandal, A.; Dana, S.; Sahoo, H.; Grandhi, G.; Baidya, M. Org. Lett. 2017, 19, 2430. |
| [21] | (a) Edwards, J. T.; Merchant, R. R.; McClymont, K. S.; Knouse, K. W.; Qin, T.; Malins, L. R.; Vokits, B.; Shaw, S. A.; Bao, D.-H.; Wei, F.-L.; Zhou, T.; Eastgate, M. D.; Baran, P. S. Nature 2017, 545, 213. |
| [21] | (b) Myers, A. G.; Tanaka, D.; Mannion, M. R. J. Am. Chem. Soc. 2002, 124, 11250. |
| [22] | (a) Huang, L.; Olivares, A. M.; Weix, D. J. Angew. Chem., Int. Ed. 2017, 56, 11901. |
| [22] | (b) Han, S.; Kim, H.-S.; Zhang, M.; Xia, Y.; Lee, S. Org. Lett. 2019, 21, 5426. |
| [23] | (a) Chatupheeraphat, A.; Liao, H.-H.; Srimontree, W.; Guo, L.; Minenkov, Y.; Poater, A.; Cavallo, L.; Rueping, M. J. Am. Chem. Soc. 2018, 140, 3724. |
| [23] | (b) Wang, D.; Yuan, Z.; Liu, Q.; Chen, P.; Liu, G. Chin. J. Chem. 2018, 36, 507. |
| [23] | (c) Ye, W.; Yan, Z.; Wan, C.; Hou, H.; Wang, Z. Acta Chim. Sinica 2018, 76, 99. (in Chinese) |
| [23] | (叶文波, 晏子聪, 万常峰, 侯豪情, 汪志勇, 化学学报, 2018, 76, 99.) |
| [23] | (d) Song, Z.; Jiang, L.; Yi, W. Acta Chim. Sinica 2018, 76, 967. (in Chinese) |
| [23] | (宋治东, 蒋绿齐, 易文斌, 化学学报, 2018, 76, 967.) |
| [24] | (a) Goossen, L. J.; Deng, G.; Levy, L. M. Science 2006, 313, 662. |
| [24] | (b) Moon, P. J.; Fahandej-Sadi, A.; Qian, W.; Lundgren, R. J. Angew. Chem., Int. Ed. 2018, 57, 4612. |
| [25] | (a) Wang, Z.; Guo, C.-Y.; Yang, C.; Chen, J.-P. J. Am. Chem. Soc. 2019, 141, 5617. |
| [25] | (b) Huang, X.; Liu, W.; Hooker, J. M.; Groves, J. T. Angew. Chem., Int. Ed. 2015, 54, 5241. |
| [25] | (c) Yin, X.; Jiang, Y.; Chu, S.; Weng, G.; Fang, X.; Pan, Y. Acta Chim. Sinica 2018, 76, 436. (in Chinese) |
| [25] | (尹欣驰, 江游, 楚士颖, 翁国锋, 方向, 潘远江, 化学学报, 2018, 76, 436.) |
| [26] | Maehara, A.; Tsurugi, H.; Satoh, T.; Miura, M. Org. Lett. 2008, 10, 1159. |
| [27] | Yamashita, M.; Horiguchi, H.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2009, 74, 7481. |
| [28] | Wang, C.; Rakshit, S.; Glorius, F. J. Am. Chem. Soc. 2010, 132, 14006. |
| [29] | Mochida, S.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2010, 12, 5776. |
| [30] | Mochida, S.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2011, 76, 3024. |
| [31] | Yugandar, S.; Morita, T.; Nakamura, H. Org. Biomol. Chem. 2020, 18, 8625. |
| [32] | Kim, K.; Vasu, D.; Im, H.; Hong, S. Angew. Chem., Int. Ed. 2016, 55, 8652. |
| [33] | Bhunia, A.; Studer, A. ACS Catal. 2018, 8, 1213. |
| [34] | Mandal, A.; Sahoo, H.; Dana, S.; Baidya, M. Org. Lett. 2017, 19, 4138. |
| [35] | Kumar, N. Y. P.; Rogge, T.; Yetra, S. R.; Bechtoldt, A.; Clot, E.; Ackermann, L. Chem.-Eur. J. 2017, 23, 17449. |
| [36] | Pu, F.; Liu, Z.-W.; Zhang, L.-Y.; Fan, J.; Shi, X.-Y. ChemCatChem 2019, 11, 4116. |
| [37] | Bettadapur, K. R.; Lanke, V.; Prabhu, K. R. Chem. Commun. 2017, 53, 6251. |
| [38] | Kumar, N. Y. P.; Bechtoldt, A.; Raghuvanshi, K.; Ackermann, L. Angew. Chem., Int. Ed. 2016, 55, 6929. |
| [39] | Huang, L.; Biafora, A.; Zhang, G.; Bragoni, V.; Goossen, L. J. Angew. Chem., Int. Ed. 2016, 55, 6933. |
| [40] | Biafora, A.; Khan, B. A.; Bahri, J.; Hewer, J. M.; Goossen, L. J. Org. Lett. 2017, 19, 1232. |
| [41] | Zhang, J.; Shrestha, R.; Hartwig, J. F.; Zhao, P. Nat. Chem. 2016, 8, 1144. |
| [42] | Spencer, A. R. A.; Korde, R.; Font, M.; Larrosa, I. Chem. Sci. 2020, 11, 4204. |
| [43] | Shi, X.-Y.; Renzetti, A.; Kundu, S.; Li, C.-J. Adv. Synth. Catal. 2014, 356, 723. |
| [44] | Shi, X.-Y.; Liu, K.-Y.; Fan, J.; Dong, X.-F.; Wei, J.-F.; Li, C.-J. Chem.-Eur. J. 2015, 21, 1900. |
| [45] | Shi, X.-Y.; Dong, X.-F.; Fan, J.; Liu, K.-Y.; Wei, J.-F.; Li, C.-J. Sci. China: Chem. 2015, 58, 1286. |
| [46] | Tulichala, R. N. P.; Shankar, M.; Swamy, K. C. K. J. Org. Chem. 2018, 83, 4375. |
| [47] | Xie, Z.; Quan, Y. J. Am. Chem. Soc. 2014, 136, 15513. |
| [48] | Jin, X.-Y.; Xie, L.-J.; Cheng, H.-P.; Liu, A.-D.; Li, X.-D.; Wang, D.; Cheng, L.; Liu, L. J. Org. Chem. 2018, 83, 7514. |
| [49] | Nandi, D.; Jhou, Y.-M.; Lee, J.-Y.; Kuo, B.-C.; Liu, C.-Y.; Huang, P.-W.; Lee, H. M. J. Org. Chem. 2012, 77, 9384. |
| [50] | Tang, J.; Hackenberger, D.; Goossen, L. J. Angew. Chem., Int. Ed. 2016, 55, 11296. |
| [51] | Cornella, J.; Righi, M.; Larrosa, I. Angew. Chem., Int. Ed. 2011, 50, 9429. |
| [52] | Luo, J.; Preciado, S.; Larrosa, I. Chem. Commun. 2015, 51, 3127. |
| [53] | Luo, J.; Preciado, S.; Larrosa, I. J. Am. Chem. Soc. 2014, 136, 4109. |
| [54] | Font, M.; Spencer, A. R. A.; Larrosa, I. Chem. Sci. 2018, 9, 7133. |
| [55] | Hu, T.; Xu, K.; Ye, Z.; Zhu, K.; Wu, Y.; Zhang, F. Org. Lett. 2019, 21, 7233. |
| [56] | Ye, Z.; Li, Y.; Xu, K.; Chen, N.; Zhang, F. Org. Lett. 2019, 21, 9869. |
| [57] | Pu, F.; Zhang, L.-Y.; Liu, Z.-W.; Shi, X.-Y. Adv. Synth. Catal. 2018, 360, 2644. |
| [58] | Agasti, S.; Dey. A.; Maiti, D. Chem. Commun. 2016, 52, 12191. |
| [59] | Zhang, Y.; Zhao, H.; Zhang, M.; Su, W. Angew. Chem., Int. Ed. 2015, 54, 3817. |
| [60] | Qin, X.; Sun, D.; You, Q.; Cheng, Y.; Lan, J.; You, J. Org. Lett. 2015, 17, 1762. |
| [61] | Han, F.; Xun, S.; Jia, L.; Zhang, Y.; Zou, L.; Hu, X. Org. Lett. 2019, 21, 5907. |
| [62] | Bhadra, S.; Dzik, W. I.; Goossen, L. J. Angew. Chem., Int. Ed. 2013, 52, 2959. |
| [63] | Fu, Z.; Jiang, Y.; Wang, S.; Song, Y.; Guo, S.; Cai, H. Org. Lett. 2019, 21, 3003. |
| [64] | Gao, Y.; Nie, J.; Li, Y.; Li, X.; Chen, Q.; Huo, Y.; Hu, X. Org. Lett. 2020, 22, 2600. |
/
| 〈 |
|
〉 |