

吲哚氮杂环作为自由基受体在串联环化反应中的研究进展
收稿日期: 2021-07-05
修回日期: 2021-09-01
网络出版日期: 2021-09-08
基金资助
江苏省高校自然科学基金(19KJB150020)
Research Progress in Radical Cascade Reaction Using Nitrogen Heterocycle in Indoles as Radical Acceptors
Received date: 2021-07-05
Revised date: 2021-09-01
Online published: 2021-09-08
Supported by
Natural Science Foundation of the Jiangsu Higher Education Institutions of China(19KJB150020)
王弯弯 , 张明明 , 杨文超 , 杨小虎 . 吲哚氮杂环作为自由基受体在串联环化反应中的研究进展[J]. 有机化学, 2022 , 42(1) : 75 -84 . DOI: 10.6023/cjoc202107012
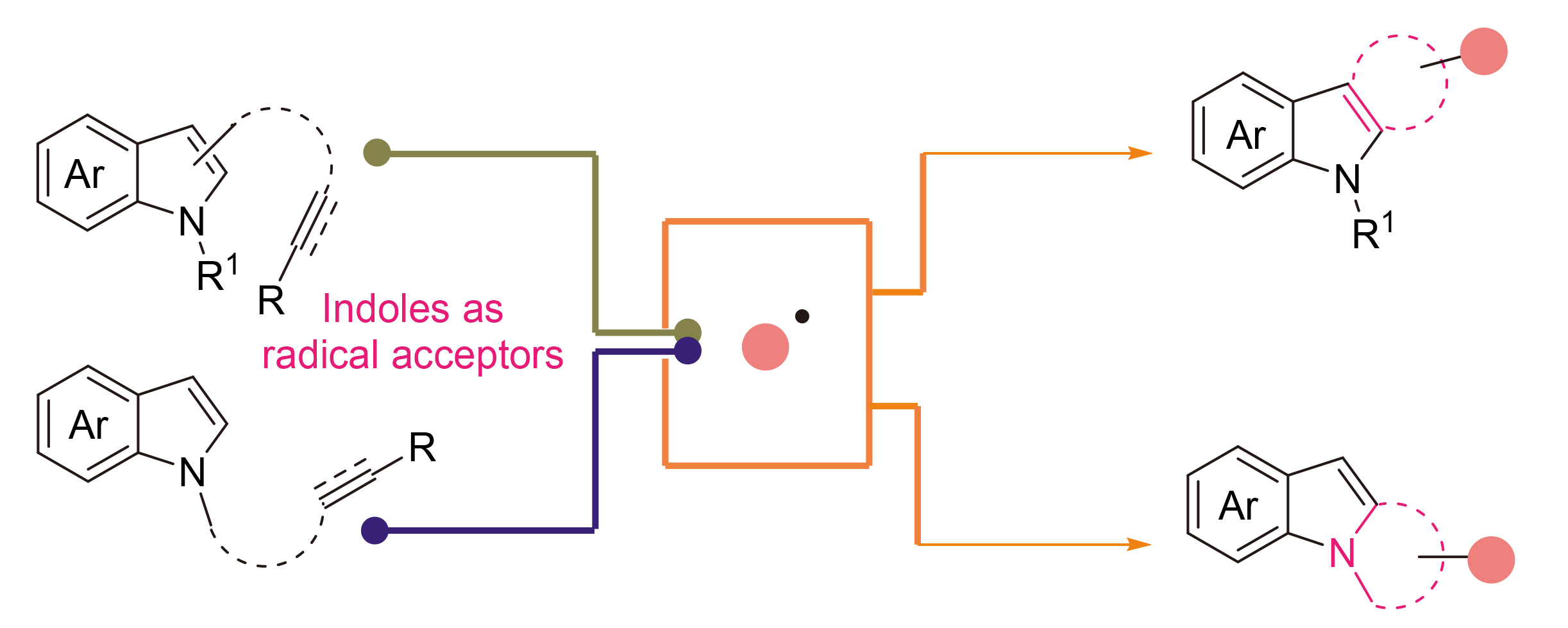
Indole skeleton has been widely utilized in the synthesis and late-stage modification of biologically active compounds, especially in the fields of medicines and pesticides. In recent years, constructing spiro compounds by asymmetric dearomatization of indoles has become extremely promising. Meanwhile, the radical tandem reaction of unsaturated bonds has been a significant branch of organic chemistry, and this methodology has become a crucial approach to modify the indole skeleton. Therefore, the recent progress in the field of radical cascade reaction of unsaturated hydrocarbon using nitrogen heterocycle in indoles as radical acceptors is summarized. Besides, the reaction design, mechanism and prospects of this field are also discussed.
| [1] | (a) Oishi, S.; Watanabe, T.; Sawada, J.; Asai, A.; Ohno, H.; Fujii, N. J. Med. Chem. 2010, 53, 5054. |
| [1] | (b) Mahmoud, M. M.; Ali, H. I.; Ahn, K. H.; Damaraju, A.; Samala, S.; Pulipati, V. K.; Kolluru, S.; Kendall, D. A.; Lu, D. J. Med. Chem. 2013, 56, 7965. |
| [2] | (a) Xiao, Z.; Wang, L.; Wei, J.; Ran, C.; Liang, S.; Shang, J.; Chen, G.-Y.; Zheng, C. Chem. Commun. 2020, 56, 4164. |
| [2] | (b) Wang, D., https://mp.weixin.qq.com/s/U8FVSkdON-eKo7rUcu7kQw. |
| [2] | (c) Shi, Z.; Nie, K.; Liu, C.; Zhang, M.; Zhang, W. Chin. J. Org. Chem. 2020, 40, 327. (in Chinese) |
| [2] | (施展, 聂克睿, 刘畅, 张明智, 章维华, 有机化学, 2020, 40, 327.) |
| [3] | (a) Huang, G.; Yin, B. Adv. Synth. Catal. 2019, 361, 405. |
| [3] | (b) Lü, S.; Zhang, G.; Chen, J.; Gao, W. Adv. Synth. Catal. 2020, 362, 462. |
| [4] | (a) Yang, W.-C.; Feng, J.-G.; Wu, L.; Zhang, Y.-Q. Adv. Synth. Catal. 2019, 361, 1700. |
| [4] | (b) Yang, W.-C.; Zhang, M.-M.; Feng, J.-G. Adv. Synth. Catal. 2020, 362, 4446. |
| [4] | (c) Shang, T.; Lu, L.; Cao, Z.; Liu, Y.; He, W.; Yu, B. Chem. Commun. 2019, 55, 5408. |
| [4] | (d) Wu, Y.; Chen, J.-Y.; Li, Q.; Wei, W.-T. Chin. J. Org. Chem. 2020, 40, 589. (in Chinese) |
| [4] | (吴燕, 陈锦杨, 李强, 魏文廷, 有机化学, 2020, 40, 589.) |
| [4] | (e) Zhang, M.; Shen, L.; Dong, S.; Li, B.; Meng, F.; Si, W.; Yang, W. Eur. J. Org. Chem. 2021, 31, 4465. |
| [5] | (a) Zhang, B.; Studer, A. Chem. Soc. Rev. 2015, 44, 3505. |
| [5] | (b) Lei, J.; Huang, J.; Zhu, Q. Org. Biomol. Chem. 2016, 14, 2593. |
| [5] | (c) Song, B.; Xu, B. Chem. Soc. Rev. 2017, 46, 1103. |
| [5] | (d) Yang, W.-C.; Wei, K.; Sun, X.; Zhu, J.; Wu, L. Org. Lett. 2018, 20, 3144. |
| [5] | (e) Liu, Y.; Chen, X.-L.; Sun, K.; Li, X.-Y.; Zeng, F.-L.; Liu, X.-C.; Qu, L.-B.; Zhao, Y.-F.; Yu, B. Org. Lett. 2019, 21, 4019. |
| [6] | He, Z.; Bae, M.; Wu, J.; Jamison, T. F. Angew. Chem., Int. Ed. 2014, 53, 14451. |
| [7] | Han, G.; Wang, Q.; Chen, L.; Liu, Y.; Wang, Q. Adv. Synth. Catal. 2016, 358, 561. |
| [8] | (a) Yang, W.; Yang, S.; Li, P.; Wang, L. Chem. Commun. 2015, 51, 7520. |
| [8] | (b) Chen, Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J. Sci. China Chem. 2019, 62, 24. |
| [8] | (c) Liu, Y.; Lin, L.; Han, Y.; Liu, Y. Chin. J. Org. Chem. 2020, 40, 4216. (in Chinese) |
| [8] | (刘洋, 林立青, 韩莹徽, 刘颖杰, 有机化学, 2020, 40, 4216.) |
| [8] | (d) Yang, W.; Zhang, M.; Chen, W.; Yang, X.; Feng, J. Chin. J. Org. Chem. 2020, 40, 4060. (in Chinese) |
| [8] | (杨文超, 张明明, 陈旺, 杨小虎, 冯建国, 有机化学, 2020, 40, 4060.) |
| [8] | (e) He, S.-Q.; Li, H.-C.; Chen, X.-L.; Krylov, I. B.; Terent’ev, A. O. Qu, L.-B.; Yu, B. Chin. J. Org. Chem. 2021, 41, 4661. (in Chinese) |
| [8] | (贺帅旗, 李昊聪, 陈晓岚, Igor B. Krylov, Alexander O. Terent'ev, 屈凌波, 於兵, 有机化学, 2021, 41, 4661.) |
| [8] | (f) Yang, W.-C.; Zhang, M.-M.; Sun, Y.; Chen, C.-Y.; Wang, L. Org. Lett. 2021, 23, 6691. |
| [9] | Zhu, M.; Zhou, K.; Zhang, X.; You, S.-L. Org. Lett. 2018, 20, 4379. |
| [10] | Qin, W.-B.; Xiong, W.; Li, X.; Chen, J.-Y.; Lin, L.-T.; Wong, H. N. C.; Liu, G.-K. J. Org. Chem. 2020, 85, 10479. |
| [11] | Liu, Y.; Chen, Z.; Wang, Q.-L.; Chen, P.; Xie, J.; Xiong, P.-Q.; Zhang, P.-L.; Tang, K.-W. J. Org. Chem. 2020, 85, 2385. |
| [12] | (a) Penteado, F.; Lopes, E. F.; Alves, D.; Perin, G.; Jacob, R. G.; Lenardão, E. J. Chem. Rev. 2019, 119, 7113. |
| [12] | (b) Yang, W.-C.; Dai, P.; Luo, K.; Ji, Y.-G.; Wu, L. Adv. Synth. Catal. 2017, 359, 2390. |
| [12] | (c) Zhu, H.-L.; Zeng, F.-L.; Chen, X.-L.; Sun, K.; Li, H.-C.; Yuan, X.-Y.; Qu, L.-B.; Yu, B. Org. Lett. 2021, 23, 2976. |
| [12] | (d) Ji, W.; Tan, H.; Wang, M.; Li, P.; Wang, L. Chem. Commun. 2016, 52, 1462. |
| [12] | (e) Xu, N.; Li, P.; Xie, Z.; Wang, L. Chem.-Eur. J. 2016, 22, 2236. |
| [12] | (f) Li, D.; Wang, M.; Liu, J.; Zhao, Q.; Wang, L. Chem. Commun. 2013, 49, 3640. |
| [12] | (g) Reddy, C. R.; Kajareab, R. C.; Punna, N. Chem. Commun. 2020, 56, 3445. |
| [13] | (a) Zhang, Y.; Sun, K.; Lü, Q.; Chen, X.; Qu, L.; Yu, B. Chin. Chem. Lett. 2019, 30, 1361. |
| [13] | (b) Xu, J.; Zhang, S.; Luo, Y.; Zhang, L.; Zhang, F.; Huang, T.; Song, Q. Acta Chim. Sinica 2019, 77, 932. (in Chinese) |
| [13] | (许健, 张世樊, 罗莹, 张荔, 张帆, 黄挺菁, 宋秋玲, 化学学报, 2019, 77, 932.) |
| [13] | (c) Liu, X.-C.; Chen, X.-L.; Liu, Y.; Sun, K.; Peng, Y.-Y.; Qu, L.-B.; Yu, B. ChemSusChem 2020, 13, 298. |
| [14] | Li, C.; Xue, L.; Zhou, J.; Zhao, Y.; Han, G.; Hou, J.; Song, Y.; Liu, Y. Org. Lett. 2020, 22, 3291. |
| [15] | (a) Luo, K.; Yang, W.-C.; Wu, L. Asian J. Org. Chem. 2017, 6, 350. |
| [15] | (b) Jing, C.; Chen, X.; Sun, K.; Yang, Y.; Chen, T.; Liu, Y.; Qu, L.; Zhao, Y.; Yu, B. Org. Lett. 2019, 21, 486. |
| [15] | (c) Zhou, Z.-Z.; Jin, D.-P.; Li, L.-H.; He, Y.-T.; Zhou, P.-X.; Yan, X.-B.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2014, 16, 5616. |
| [15] | (d) Li, Y.-M.; Sun, M.; Wang, H.-L.; Tian, Q.-P.; Yang, S.-D. Angew. Chem., Int. Ed. 2013, 52, 3972. |
| [16] | (a) Liu, J. M.; Zhao, S. S.; Song, W. W.; Li, R.; Guo, X. Y.; Zhuo, K. L.; Yue, Y. Y. Adv. Synth. Catal. 2017, 359, 609. |
| [16] | (b) Xu, J.; Yu, X.; Song, Q. Org. Lett. 2017, 19, 980. |
| [17] | Gorre, R.; Enagandhula, D.; Balasubramanianbc, S.; Akondi, S. M. Org. Biomol. Chem. 2020, 18, 1354. |
| [18] | (a) Chen, S.; Zhang, P. B.; Shu, W. Y.; Gao, Y. Z.; Tang, G.; Zhao, Y. F. Org. Lett. 2016, 18, 5712. |
| [18] | (b) Zhang, H.; Li, W.; Zhu, C. J. Org. Chem. 2017, 82, 2199. |
| [19] | Hua, H.-L.; Zhang, B.-S.; He, Y.-T.; Qiu, Y.-F.; Wu, X.-X.; Xu, P.-F.; Liang, Y.-M. Org. Lett. 2016, 18, 216. |
| [20] | (a) Zhu, J.; Yang, W.-C.; Wang, X.-D.; Wu, L. Adv. Synth. Catal. 2018, 360, 386. |
| [20] | (b) Zhang, M.-M.; Sun, Y.; Wang, W.-W.; Chen, K.-K.; Yang, W.-C.; Wang, L. Org. Biomol. Chem. 2021, 19, 3844. |
| [20] | (c) Zhang, P.; Gao, Y.; Chen, S.; Tang, G.; Zhao, Y. Org. Chem. Front. 2017, 4, 1350. |
| [20] | (d) Xie, X.; Li, P.; Wang, L. Eur. J. Org. Chem. 2019, 221. |
| [20] | (e) Chen, H.; Liu, M.; Qiu, G.; Wu, J. Adv. Synth. Catal. 2019, 361, 146. |
| [20] | (f) Zhang, P.; Shi, S.; Gao, X.; Han, S.; Lin, J.; Zhao, Y. Org. Biomol. Chem. 2019, 17, 2873. |
| [20] | (g) Shen, Z.-J.; Huang, B.; Ma, N.; Yao, L.; Yang, C.; Guo, L.; Xia, W. Adv. Synth. Catal. 2021, 363, 1944. |
| [21] | Zhu, J.; Sun, S.; Xia, M.; Gu, N.; Cheng, J. Org. Chem. Front. 2017, 4, 2153. |
| [22] | Zhu, X.-Y.; Han, Y.-P.; Li, M.; Li, X.-S.; Liang, Y.-M. Adv. Synth. Catal. 2018, 360, 3460. |
| [23] | Gharpure, S. J.; Shelke, Y. G. Org. Lett. 2017, 19, 5022. |
| [24] | (a) Ho, H. E.; Pagano, A.; Rossi-Ashton, J. A.; Donald, J. R.; Epton, R. G.; Churchill, J. C.; James, M. J.; O'Brien, P.; Taylor, R. J. K.; Unsworth, W. P. Chem. Sci. 2020, 11, 1353. |
| [24] | (b) Zhou, X.-J.; Liu, H.-Y.; Mo, Z.-Y.; Ma, X.-L.; Chen, Y.-Y.; Tang, H.-T.; Pan, Y.-M.; Xu, Y.-L. Chem.-Asian J. 2020, 15, 1536. |
| [25] | Sun, K.; Chen, X. L.; Zhang, Y. L.; Li, K.; Huang, X. Q.; Peng, Y. Y.; Qu, L. B.; Yu, B. Chem. Commun. 2019, 55, 12615. |
/
| 〈 |
|
〉 |