

碘(III)介导的碳自由基参与的氧化偶联反应
收稿日期: 2021-09-06
修回日期: 2021-10-11
网络出版日期: 2021-11-03
基金资助
国家自然科学基金(21602029); 安徽省高校优秀人才支持计划重点(gxyqZD2020030); 安徽省教育厅自然科学重点(KJ2020A0526); 阜阳市政府-阜阳师范学院横向合作科研(XDHX201722); 安徽省科技重大专项(18030701213)
Iodine(III)-Promoted Oxidative Cross-Coupling Reactions of C—H Bonds via a Free Radical Process
Received date: 2021-09-06
Revised date: 2021-10-11
Online published: 2021-11-03
Supported by
National Natural Science Foundation of China(21602029); Natural Science Foundation of Higher Education Institutions in Anhui Province(gxyqZD2020030); Key Projects of the Support Program for Outstanding Young Talents in Anhui Province Colleges and Universities(KJ2020A0526); Horizontal Cooperation Project of Fuyang Municipal Government(XDHX201722); Major Science and Technology Projects of Anhui Province(18030701213)
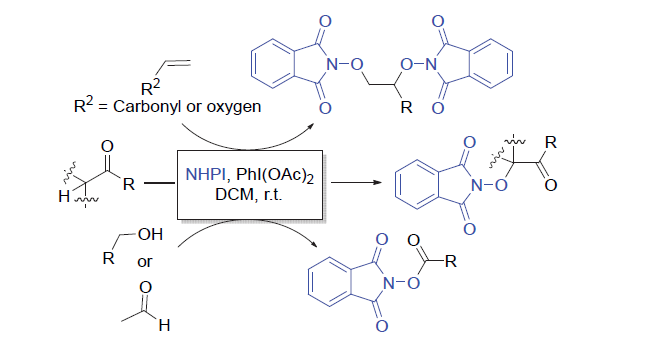
氧取代N-羟基邻苯二甲酰亚胺衍生物广泛存在于天然产物和药物中, 其合成具有重要的意义. 大多数烯烃、酮、酯、醛和醇的C=C/C—H键的活化方法通常需要用到金属或过氧化物. 以廉价安全的醋酸碘苯作为脱氢剂, N-羟基邻苯二甲酰亚胺为自由基前体, 实现了烯烃的双氧化、羰基α位氢的氧化、醛氢氧化及伯醇的氧化酯化反应. 该方法主要通过自由基机理, 具有环境友好、产率高及底物适用范围广等优点. 反应体系简单实用, 可以很好地应用于氧取代N-羟基邻苯二甲酰亚胺衍生物的合成.
吴福芳 , 李雪健 , 贾浩 , 韩宣振 , 沈晓宝 . 碘(III)介导的碳自由基参与的氧化偶联反应[J]. 有机化学, 2022 , 42(3) : 884 -890 . DOI: 10.6023/cjoc202109012
The synthesis of oxygen-substituted N-hydroxy phthalimide derivatives is essential due to their ubiquity in natural products and pharmaceuticals. Metals or peroxides are often required for most C=C/C—H bond activation methods used for olefins, ketones, esters, aldehydes and alcohols. Using cheap and safe iodobenzene diacetate as a feasible dehydrogenation agent and N-hydroxy phthalimide as free radical precursor, the dioxidation of olefins, α-oxidation of carbonyl compounds, oxidation of aldehydes, and oxidative esterification of primary alcohols were successfully realized. This method occurs via a radical mechanism and has the characteristics of mild metal-free reaction conditions, good compatibility and wide substrate scope.

| [1] | Wilson, R. M.; Stockdill, J. L.; Wu, X.; Li, X.; Vadola, P. A.; Park, P. K.; Wang, P.; Danishefsky, S. J. Angew. Chem., Int. Ed. 2012, 51, 2834. |
| [2] | (a) Brown, M. F.; Mitton-Fry, M. J.; Arcari, J. T.; Barham, R.; Casavant, J.; Gerstenberger, B. S.; Han, S.; Hardink, J. R.; Harris, T. M.; Hoang, T.; Huband, M. D.; Lall, M. S.; Lemmon, M. M.; Li, C.; Lin, J.; McCurdy, S. P.; McElroy, E.; McPherson, C.; Marr, E. S.; Mueller, J. P.; Mullins, L.; Nikitenko, A. A.; Noe, M. C.; Penzien, J.; Plummer, M. S.; Schuff, B. P.; Shanmugasundaram, V.; Starr, J. T.; Sun, J.; Tomaras, A.; Young, J. A.; Zaniewski, R. P. J. Med. Chem. 2013, 56, 5541. |
| [2] | (b) Sharma, G. V. M.; Manohar, V.; Dutta, S. K.; Sridhar, B.; Ramesh, V.; Srinivas, R.; Kunwar, A. C. J. Org. Chem. 2010, 75, 1087. |
| [2] | (c) Yamawaki, K.; Nomura, T.; Yasukata, T.; Tanimoto, N.; Uotani, K.; Miwa, H.; Yamano, Y.; Takeda, K.; Nishitani, Y. Bioorg. Med. Chem. 2008, 16, 1632. |
| [3] | Ishii, T.; Kakeno, Y.; Nagao, K.; Ohmiya, H. J. Am. Chem. Soc. 2019, 141, 3854. |
| [4] | (a) Allen, L. J.; Cabrera, P. J.; Lee, M.; Sanford, M. S. J. Am. Chem. Soc. 2014, 136, 5607. |
| [4] | (b) Qin, T.; Malins, L. R.; Edwards, J. T.; Merchant, R. R.; Novak, A. J.; Zhong, J. Z.; Mills, R. B.; Yan, M.; Yuan, C.; Eastgate, M. D.; Baran, P. S. Angew. Chem., Int. Ed. 2017, 56, 260. |
| [4] | (c) Candish, L.; Teders, M.; Glorius, F. J. Am. Chem. Soc. 2017, 139, 7440. |
| [4] | (d) Montesinos-Magraner, M.; Costantini, M.; Ramirez-Contreras, R.; Muratore, M. E.; Johansson, M. J.; Mendoza, A. Angew. Chem., Int. Ed. 2019, 58, 5930. |
| [4] | (e) Shu, C.; Noble, A.; Aggarwal, V. K. Nature 2020, 586, 714. |
| [5] | (a) Xia, X. F.; Zhu, S. L.; Gu, Z.; Wang, H.; Li, W.; Liu, X.; Liang, Y. M. J. Org. Chem. 2015, 80, 5572. |
| [5] | (b) Xia, X.-F.; Zhu, S.-L.; Zhang, D. Tetrahedron 2015, 71, 8517. |
| [5] | (c) Tang, S. Q.; Wang, A. P.; Schmitt, M.; Bihel, F. Tetrahedron Lett. 2018, 59, 1465. |
| [5] | (d) Krylov, I. B.; Paveliev, S. A.; Matveeva, O. K.; Terent'ev, A. O. Tetrahedron 2019, 75, 2529. |
| [6] | (a) Tan, B.; Toda, N.; Barbas, C. F. Angew. Chem., Int. Ed. 2012, 51, 12538. |
| [6] | (b) Dinda, M.; Bose, C.; Ghosh, T.; Maity, S. RSC Adv. 2015, 2015, 44928. |
| [6] | (c) Siddaraju, Y.; Prabhu, K. R. Org. Biomol. Chem. 2015, 13, 11651. |
| [6] | (d) Lv, Y.; Sun, K.; Pu, W.; Mao, S.; Li, G.; Niu, J.; Chen, Q.; Wang, T. RSC Adv. 2016, 2016, 93486. |
| [6] | (e) Su, W.; Jin, C.; Guo, Z.; Jiang, X.; Zhou, J.; Sun, B. Synlett 2017, 28, 1321. |
| [6] | (f) Xu, X.; Li, P.; Huang, Y.; Tong, C.; Yan, Y.; Xie, Y. Tetrahedron Lett. 2017, 58, 1742. |
| [6] | (g) Xu, X.; Sun, J.; Lin, Y.; Cheng, J.; Li, P.; Jiang, X.; Bai, R.; Xie, Y. Eur. J. Org. Chem. 2017, 2017, 7160. |
| [6] | (h) Feizpour, F.; Jafarpour, M.; Rezaeifard, A. New J. Chem. 2018, 42, 807. |
| [6] | (i) Joo, S.-R.; Kim, J.-S.; Park, S.-Y.; Kim, S.-H. Bull. Korean Chem. Soc. 2018, 39, 829. |
| [6] | (j) Sun, B.; Wang, Y.; Li, D.; Jin, C.; Su, W. Org. Biomol. Chem. 2018, 16, 2902. |
| [6] | (k) Krylov, I. B.; Lopat'eva, E. R.; Budnikov, A. S.; Nikishin, G. I.; Terent'ev, A. O. J. Org. Chem. 2020, 85, 1935. |
| [6] | (l) Chen, R.; Liu, B.; Li, W.; Wang, K.-K.; Miao, C.; Li, Z.; Lv, Y.; Liu, L. RSC Adv. 2021, 11, 8051. |
| [7] | (a) Singha, K.; Ghosh, S. C.; Panda, A. B. Chem.-Asian J. 2019, 14, 3205. |
| [7] | (b) Singha, K.; Ghosh, S. C.; Panda, A. B. Eur. J. Org. Chem. 2021, 2021, 657. |
| [8] | Wu, F. F.; Han, X. Z.; Li, X. J.; Shen, X. B.; Wang, C.; Tian, Z. M.; Cheng, B.; Zhang, J. B.; Sheng, L. Q.; Zhai, H. B. Commun. Chem. 2021, 4, 46. |
| [9] | Tamura, Y.; Yakura, T.; Haruta, J.; Kita, Y. J. Org. Chem. 1987, 52, 3927. |
| [10] | Kita, Y.; Tohma, H.; Hatanaka, K.; Takada, T.; Fujita, S.; Mitoh, S.; Sakurai, H.; Oka, S. J. Am. Chem. Soc. 1994, 116, 3684. |
| [11] | Qian, P.-C.; Liu, Y.; Song, R.-J.; Hu, M.; Yang, X.-H.; Xiang, J.-N.; Li, J.-H. Eur. J. Org. Chem. 2015, 2015, 1680. |
| [12] | Bag, R.; Sar, D.; Punniyamurthy, T. Org. Lett. 2015, 17, 2010. |
| [13] | Andia, A. A.; Miner, M. R.; Woerpel, K. A. Org. Lett. 2015, 17, 2704. |
| [14] | Huihui, K. M.; Caputo, J. A.; Melchor, Z.; Olivares, A. M. J. Am. Chem. Soc. 2016, 138, 5016. |
| [15] | Chan, C.-M.; Xing, Q.; Chow, Y.-C.; Hung, S.-F.; Yu, W.-Y. Org. Lett. 2019, 21, 8037. |
| [16] | Zhao, W.; Wurz, R. P.; Peters, J. C.; Fu, G. C. J. Am. Chem. Soc. 2017, 139, 12153. |
| [17] | Cheng, W. M.; Shang, R.; Zhao, B.; Xing, W. L.; Fu, Y. Org. Lett. 2017, 19, 4291. |
/
| 〈 |
|
〉 |