

基于原位捕获异腈的Ugi四组分反应及其后修饰串联反应: 一锅法合成含氮杂环化合物
收稿日期: 2021-08-18
修回日期: 2021-10-08
网络出版日期: 2021-11-10
基金资助
国家自然科学基金(21602123); 高等学校学科创新引智计划(111计划)(D20015)
Ugi Four-Component Reaction Based on in-situ Capture of Isocyanide and Post-Modification Tandem Reaction: One-Pot Synthesis of Nitrogen Heterocycles
Received date: 2021-08-18
Revised date: 2021-10-08
Online published: 2021-11-10
Supported by
National Natural Science Foundation of China(21602123); Program of Introducing Talents of Discipline to Universities (111 Project)(D20015)
罗享豪 , 谢益碧 , 黄年玉 , 王龙 . 基于原位捕获异腈的Ugi四组分反应及其后修饰串联反应: 一锅法合成含氮杂环化合物[J]. 有机化学, 2022 , 42(3) : 838 -846 . DOI: 10.6023/cjoc202108030
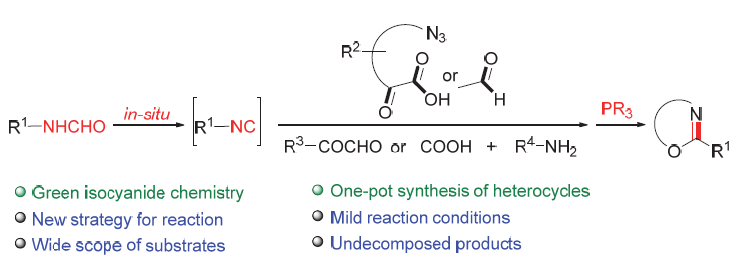
A novel strategy of Ugi four-component and post-modification tandem reaction based on in-situ capture of isocyanide is reported in this paper. According to this reaction strategy, the isocyanide was prepared in situ and immediately captured to participate in Ugi four-component reaction and the subsequent modification tandem reaction. Compared with the previous reports, based on in-situ capture of isocyanide was realized in the Ugi four-component reaction and post-modification tandem reaction, which solved the problems of isocyanide instability, high toxicity and unfriendly to the environment in the reaction. Mild reaction system conditions and simplified preparation method provide a novel green and pollution-free strategy for the tandem reaction of Ugi four-component and post modification series reaction with isocyanide.
| [1] | (a) Ugi, I.; Dömling, A.; Hörl, W. Endeavour 1994, 18, 115. |
| [1] | (b) Do?mling, A. Chem. Rev. 2006, 106, 17. |
| [1] | (c) Domling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083. |
| [1] | (d) de Graaff, C.; Ruijter, E.; Orru, R. V. A. Chem. Soc. Rev. 2012, 41, 3969. |
| [1] | (e) Sharma, U. K.; Sharma, N.; Vachhani, D. D.; Van der Eycken, E. V. Chem. Soc. Rev. 2015, 44, 1836. |
| [1] | (f) Zarganes-Tzitzikas, T; Chandgude, A. L.; Dömling, A. Chem. Rec. 2015, 15, 981. |
| [1] | (g) Shen, C.; Wu, X.-F. Chem.-Eur. J. 2017, 23, 2973. |
| [1] | (h) Neochoritis, C. G.; Zhao, T., Do?mling, A. Chem. Rev. 2019, 119, 1970. |
| [1] | (i) Shi, Y.; Qin, Fu.-W.; Wang, J.; Yan, Y.-M. Chin. J. Org. Chem. 2021, 41, 297. (in Chinese) |
| [1] | (石瑛, 秦富文, 王捷, 闫艳梅, 有机化学, 2021, 41, 297.) |
| [1] | (j) Zhu, J. P.; Bienaymé, H. In Multicomponent Reactions, Verlag GmbH & Co. KGaA, Weinheim, 2005, pp. 1-94. |
| [2] | (a) Dömling, A., Ugi, I. Angew. Chem. Int. Ed. 2000, 39, 3168. |
| [2] | (b) Kusebauch, U.; Beck, B.; Messer, K.; Herdtweck, E.; Domling, A. Org. Lett. 2003, 5, 4021. |
| [2] | (c) Jiang, B.; Rajale, T.; Wever, W.; Tu, S.-J.; Li, G. Chem. Asian J. 2010, 5, 2318. |
| [2] | (d) Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K. Chem. Rev. 2014, 114, 8323. |
| [2] | (e) Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958. |
| [2] | (f) Ibarra, I. A.; Islas-Jácome, A.; González-Zamora, E Org. Biomol. Chem. 2018, 16, 1402. |
| [2] | (g) Zhang, Z.; You, Y.-Z., Hong, C.-Y. Macromol. Rapid Commun. 2018, 39, 1800362. |
| [2] | (h) Zhi, S.-J.; Ma, X.-M.; Zhang, W. Org. Biomol. Chem. 2019, 17, 7632. |
| [2] | (i) Zhu, J. P.; Wang, Q.; Wang, M. X. In Multicomponent Reactions in Organic Synthesis, Verlag GmbH & Co. KGaA, Weinheim, 2014, pp. 158-182. |
| [2] | (j) Orru, R. V. A; Ruijter, E. In Synthesis of Heterocycles via Multicomponent Reactions I, Springer-Verlag, Berlin Heidelberg, 2010, pp. 199-226. |
| [3] | (a) Andreana, P. R.; Liu, C.-C.; Schreiber, S. L. Org. Lett. 2004, 6, 4231. |
| [3] | (b) El Kaim, L.; Gizolme, M.; Grimaud, L. Org. Lett. 2006, 8, 5021. |
| [3] | (c) Kreye, O.; Tóth, T.; Meier, M. A. R. J. Am. Chem. Soc. 2011, 133, 1790. |
| [3] | (d) Reza Kazemizadeh, A. Curr. Org. Chem. 2012, 16, 418. |
| [3] | (e) Solleder, S. C.; Meier, M. A. R. Angew. Chem., nt. Ed. 2014, 53, 711. |
| [3] | (f) Chandgude, A. L.; Do?mling, A. Org. Lett. 2016, 18, 6396. |
| [3] | (g) Zhang, J.-H.; Niu, L.-Z.; Li, Y.; Liu, S.; Jiang, L. Chin. J. Org. Chem. 2018, 38, 1842. (in Chinese) |
| [3] | (张君辉, 牛李智, 李映, 刘思, 姜林, 有机化学, 2018, 38, 1842.) |
| [3] | (h) Moni, L.; Banfi, L.; Cartagenova, D.; Cavalli, A.; Lambruschini, C.; Martino, E.; Orru, R. V. A; Ruijter, E.; Saya, J. M.; Sgrignani, J.; Riva, R. Org. Chem. Front. 2020, 7, 380. |
| [4] | (a) Ugi, I.; Demharter, A.; Hörl, W.; Schmid, T. Tetrahedron 1996, 52, 11657. |
| [4] | (b) Pan, S.; C. List, B. Angew. Chem., nt. Ed, 2008, 47, 3622. |
| [4] | (c) Vercillo, O. E.; Andrade, C. K. Z.; Wessjohann, L. A. Org. Lett. 2008, 10, 205. |
| [4] | (d) Riva, R. Science 2018, 361, 1072. |
| [4] | (e) Wang, Q.; Wang, D.-X.; Wang, M.-X.; Zhu, J.-P. Acc. Chem. Res. 2018, 51, 1290. |
| [4] | (f) Nazeri, M. T.; Farhid, H.; Mohammadian, R.; Shaabani, A. ACS Comb. Sci. 2020, 22, 361. |
| [4] | (g) Nazeri, M. T.; Nowee, A. B.; Shaabani, A. New J. Chem. 2021, 45, 3479. |
| [4] | (h) Guo, X.-Y.; Fang, S.-J.; Qian, H.-F.; Feng, G.-F. Chin. J. Org. Chem. 2021, 41, 1703. (in Chinese) |
| [4] | (郭小燕, 方帅军, 钱红飞, 冯高峰, 有机化学, 2021, 41, 1703.) |
| [4] | (i) Hu, H. L.; L, A. N.; Z, H. J.; S, D. Q. Chin. J. Org. Chem. 2015, 35, 2162. (in Chinese) |
| [4] | (胡汉宁, 黎安玲, 张瀚匀, 石德清, 有机化学, 2015, 35, 2162.) |
| [5] | (a) He, P.; Nie, Y. B.; Wu, J, Ding, M.-W. Org. Biomol. Chem. 2011, 9, 1429. |
| [5] | (b) Tron, G. C. Eur. J. Org. Chem. 2013, 2013, 1849. |
| [5] | (c) Welsch, S. J.; Umkehrer, M.; Kalinski, C, Ross, G.; Burdack, C.; Kolb, J.; Wild, M.; Ehrlich, A.; Wessjohann, L. A. Tetrahedron Lett. 2015, 56, 1025. |
| [5] | (d) Pertejo, P.; Corres, N.; Torroba, T.; Garcia-Valverde, M. Org. Lett. 2015, 17, 612. |
| [5] | (e) Xie, H.; Liu, J. C.; Ding, M.-W. Synthesis 2016, 48, 4541. |
| [5] | (f) Snieckus, V.; Board, J. Synfacts 2017, 13, 0241. |
| [5] | (g) Xiong, J.; Wei, X.; Yan, Y.-M.; Ding, M.-W. Tetrahedron 2017, 73, 5720. |
| [5] | (h) Gu, Z. Y.; Ji, S. J. Acta Chim. Sinica. 2018, 76, 347. (in Chinese) |
| [5] | (顾正阳, 纪顺俊, 化学学报, 2018, 76, 347.) |
| [5] | (i) Xiong, J.; Wei, X.; Wan, Y. C.; Ding, M.-W. Tetrahedron 2019, 75, 1072. |
| [5] | (j) Wang, L.; Guan, Z. R.; Ding, M.-W. Org. Biomol. Chem. 2016, 14, 2413. |
| [6] | (a) Stigter, E. A.; Guo, Z.; Bon, R. S.; Wu, Y.-W. Choidas, A.; Wolf, A.; Menninger, S.; Waldmann, H.; Blankenfeldt, W.; Goody, R. S. J. Med. Chem. 2012, 55, 8330. |
| [6] | (b) Vodela, S.; Mekala, R. V. R. Danda, R. R.; Kodhati, V. Chin Chem. Lett. 2013, 24, 625. |
| [6] | (c) Zhan, Y.-Z.; Wang, B.-L.; Zhang, L.-Y.; Zhang, Y.; Zhang, Xiao.; Li, Z.-M.; Song, H.-B. Acta Chim. Sinica. 2015, 73, 1173. (in Chinese) |
| [6] | (詹益周, 王宝雷, 张丽媛, 张燕, 张晓, 李正名, 宋海斌, 化学学报, 2015, 73, 1173.) |
| [6] | (d) Doyle, K.; Lonn, H.; Kack, H.; Van de Poel, A.; Swallow, S.; Gardiner, P.; Connolly, S.; Root, J.; Wikell, C.; Dahl, G.; Stenvall, K.; Johannesson, P. J. Med. Chem. 2016, 59, 9457. |
| [6] | (e) Jadhav, A. A.; Dhanwe, V. P.; Joshi, P. G.; Khanna, P. K. Cogent Chem. 2016, 2, 1144670. |
| [6] | (f) Mor, S.; Nagoria, S.; Sindhu, S.; Khatri, M.; Sidhu, G.; Singh, V. J. Heterocycl. Chem. 2017, 54, 3282. |
| [6] | (g) Erol, M.; Celik, I.; Temiz-Arpaci, O.; Kaynak-Onurdag, F.; Okten, S. J. Biomol. Struct. Dyn. 2021, 39, 3080. |
| [7] | (a) Costantino, L.; Barlocco, D. Curr. Med. Chem. 2006, 13, 65. |
| [7] | (b) Mandrioli, R.; Mercolini, L.; Raggi, M. A. Curr. Drug Metab. 2008, 9, 827. |
| [7] | (c) Demmer, C. S.; Bunch, L. Eur. J. Org. Chem. 2015, 97, 778. |
| [7] | (d) Kvasnica, M.; Urban, M.; Dickinson, N.; J.; Sarek, J. Nat. Prod. Rep. 2015, 32, 1303. |
| [7] | (e) Norwood IV, V. M.; Huigens III, R. W. ChemBioChem 2019, 20, 2273. |
| [7] | (f) Pathania, S.; Narang, R. K.; Rawal, R. K. Eur. J. Org. Chem. 2019, 180, 486. |
| [7] | (g) Li, Y.-Y.; Zhou, M.-B.; Xu, L. Zhou, B.-X.; Rao, Y.-T.; Nie, H.; Gu, T.-T.; Zhou, J.; Liang, X.; Yin, B. S.; Zhu, W.-H.; Osuka, A.; Song, J.-X. Org. Lett. 2020, 22, 6001. |
| [7] | (h) Ashton, H. Curr. Opin. Psychiatry 2005, 18, 249. |
| [7] | (i) Votaw, V. R.; Geyer, R.; Rieselbach, M. M.; McHugh, R. K. Drug Alcohol Depen. 2019, 200, 95. |
| [7] | (j) Wang, Y.. Ph.D. Dissertation, Central China Normal University, Wuhan, 2014. (in Chinese) |
| [7] | (王英, 博士论文, 华中师范大学, 武汉, 2014.) |
| [7] | (k) Wang, L. Ph.D. Dissertation, Central China Normal University, Wuhan, 2015. (in Chinese) |
| [7] | (王龙, 博士论文, 华中师范大学, 武汉, 2015.) |
| [8] | (a) Nishikawa, T.; Urabe, D.; Tomita, M.; Tsujimoto, T.; Iwabuchi, T.; Isobe, M. Org. Lett. 2006, 8, 3263. |
| [8] | (b) El Kaim, L.; Grimaud, L.; Schiltz, A. Org. Biomol. Chem. 2009, 7, 3024. |
| [8] | (c) El Kaim, L.; Grimaud, L.; Schiltz, A. Tetrahedron Lett. 2009, 50, 5235. |
| [8] | (d) Sharma, S.; Maurya, R. A.; Min, K. I.; Jeong, G. Y.; Kim, D. P. Angew. Chem., nt. Ed. 2013, 52, 7564. |
| [8] | (e) Rostamnia, S. RSC Adv. 2015, 5, 97044. |
| [8] | (f) Neochoritis, C. G.; Stotani, S.; Mishra, B.; Do?mling, A. Org. Lett. 2015, 17, 2002. |
| [8] | (g) Muthukumar, A.; Mamillapalli, N. C.; Sekar, G. Adv. Synth. Catal. 2016, 358, 643. |
| [9] | Fe?dou, N. M.; Parsons, P. J.; Viseux, E. M. E.; Whittle, A. J. Org. Lett. 2005, 7, 3179. |
| [10] | Kruithof, A.; Ruijter, E.; Orru, R. V. A. Chem. Asian J. 2015, 10, 508. |
| [11] | Wang, Y.; Zhou, Y.; Song, Q. Chem. Commun. 2020, 56, 6106. |
| [12] | (a) Liu, N.; Chao, F.; Liu, M. G.; Huang, N. Y.; Zou, K.; Wang, L. J. Org. Chem. 2019, 84, 2366. |
| [12] | (b) Liu, M. G.; Liu, N.; Xu, W. H.; Wang, L. Tetrahedron 2019, 75, 2748. |
| [13] | (a) Wang, L.; Xie, Y. B.; Huang, N. Y.; Yan, J. Y.; Hu, W. M.; Liu, M. G.; Ding, M. W. ACS Catal. 2016, 6, 4010. |
| [13] | (b) Wang, L.; Xie, Y. B.; Huang, N. Y.; Zhang, N. N.; Li, D. J.; Hu, Y. L.; Liu, M. G.; Li, D. S. Adv. Synth. Catal. 2017, 359, 779. |
| [13] | (c) Liu, J.; Xie, Y.; Yang, Q.; Huang, N.; Wang, L. Chin. J. Org. Chem. 2021, 41, 2374. (in Chinese) |
| [13] | (刘金妮, 谢益碧, 阳青青, 黄年玉, 王龙, 有机化学, 2021, 41, 2374.) |
| [13] | (d) Liu, J. N.; Liu, N.; Yang, Q. Q.; Wang, L. Org. Chem. Front. 2021, 8, 5296. |
| [13] | (e) Tian, A. Q.; Luo, X. H.; Ren, Z. L.; Zhao, J.; Wang, L. New J. Chem. 2021, 45, 9614. |
| [13] | (f) Zhu, G. X.; Zhao, J. X.; Duan, T. B.; Wang, L.; Wang, D. W. Asian J. Org. Chem. 2021, 10, 2213. |
| [13] | (g) Yang, Q. Q.; Liu, N.; Yan, J. Y.; Ren, Z. L.; Wang, L. Asian J. Org. Chem. 2020, 9, 116. |
| [13] | (h) Ren, Z. L.; Cai, S.; Liu, Y. Y.; Xie, Y. Q.; Yuan, D.; Lei, M.; He, P.; Wang, L. J. Org. Chem. 2020, 85, 11014. |
| [14] | (a) Xie, Y. B. M. S. Thesis, China Three Gorges University, Yichang, 2017. (in Chinese) |
| [14] | (谢益碧, 硕士论文, 三峡大学, 宜昌, 2017.) |
| [14] | (b) Wang, L.; Ren, Z. L.; Ding, M.-W. J. Org. Chem. 2015, 80, 641. |
/
| 〈 |
|
〉 |