

锰催化的碳酸乙烯亚乙酯对喹唑啉酮的C—H烯丙基化
收稿日期: 2021-10-02
修回日期: 2021-10-30
网络出版日期: 2021-11-10
基金资助
国家自然科学基金(22022204); 国家自然科学基金(21633013); 国家自然科学基金(22072167)
Manganese-Catalyzed Allylation of Quinazolinones with 4-Vinyl-1,3-dioxolan-2-one via C—H Activation
Received date: 2021-10-02
Revised date: 2021-10-30
Online published: 2021-11-10
Supported by
National Natural Science Foundation of China(22022204); National Natural Science Foundation of China(21633013); National Natural Science Foundation of China(22072167)
李玉东 , 李莹 , 董亚楠 , 夏春谷 , 李跃辉 . 锰催化的碳酸乙烯亚乙酯对喹唑啉酮的C—H烯丙基化[J]. 有机化学, 2022 , 42(3) : 847 -853 . DOI: 10.6023/cjoc202110002
The ortho-allylation of quinazolinones with 4-vinyl-1,3-dioxolan-2-one via manganese catalysis has been described. A series of allylation products with potential applications have been obtained. This protocol is also highlighted by good compatibility of functional groups and excellent E/Z selectivity. This work broadens the scope of Mn-catalyzed C—C coupling reactions.
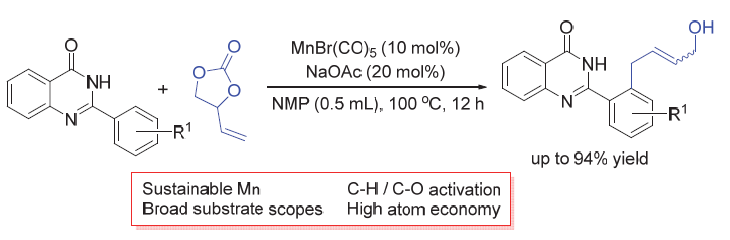
Key words: manganese-catalyzed; allylation; quinazolinones; C—H activation
| [1] | (a) Rohokale, R. S.; Kshirsagar, U. A. Synthesis 2016, 48, 1253. |
| [1] | (b) Kshirsagar, U. A. Org. Biomol. Chem. 2015, 13, 9336. |
| [1] | (c) Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Eur. J. Med. Chem. 2015, 90, 124. |
| [1] | (d) Mhaske, S. B.; Argade, N. P. Tetrahedron 2006, 62, 9787. |
| [2] | Yu, J. Q.; Shi, Z.-J. C-H Activation, Springer, Berlin, 2010. |
| [3] | (a) Nakamura, E.; Yoshikai, N. J. Org. Chem. 2010, 75, 6061. |
| [3] | (b) Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Rev. 2011, 111, 1293. |
| [3] | (c) Bauer, I.; Kno?lker, H.-J. Chem. Rev. 2015, 115, 3170-3387. |
| [4] | (a) Gao, K.; Yoshikai, N. Acc. Chem. Res. 2014, 47, 1208. |
| [4] | (b) Moselage, M.; Li, J.; Ackermann, L. ACS Catal. 2016, 6, 498. |
| [5] | Castro, L. C. M.; Chatani, N. Chem. Lett. 2015, 44, 410. |
| [6] | (a) Liu, J.; Chen, G.; Tan, Z. Adv. Synth. Catal. 2016, 358, 1174. |
| [6] | (b) Guo, X.-X.; Gu, D.-W.; Wu, Z.; Zhang, W. Chem. Rev. 2015, 115, 1622. |
| [7] | (a) Nareddy, P.; Jordan, F.; Szostak, M. ACS Catal. 2017, 7, 5721. |
| [7] | (b) Nareddy, P.; Jordan, F.; BrennerMoyer, S. E.; Szostak, M. ACS Catal. 2016, 6, 4755. |
| [7] | (c) Arockiam, P. B.; Bruneau, C.; Dixneuf, P. H. Chem. Rev. 2012, 112, 5879. |
| [7] | (d) Thirunavukkarasu, V. S.; Kozhushkov, S. I.; Ackermann, L. Chem. Commun. 2014, 50, 29. |
| [8] | (a) Piou, T.; Rovis, T. Acc. Chem. Res. 2018, 51, 170. |
| [8] | (b) Song, G.; Wang, F.; Li, X. Chem. Soc. Rev. 2012, 41, 3651. |
| [8] | (c) Kuhl, N.; Schro?der, N.; Glorius, F. Adv. Synth. Catal. 2014, 356, 1443. |
| [8] | (d) Song, G.; Li, X. Acc. Chem. Res. 2015, 48, 1007. |
| [9] | (a) Daugulis, O.; Do, H.-Q.; Shabashov, D. Acc. Chem. Res. 2009, 42, 1074. |
| [9] | (b) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147. |
| [9] | (c) He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q. Chem. Rev. 2017, 117, 8754. |
| [10] | (a) Kuninobu, Y.; Nishina, Y.; Takeuchi, T.; Takai, K. Angew. Chem., nt. Ed. 2007, 46, 6518. |
| [10] | (b) Hu, Y.; Wang, C. ChemCatChem 2019, 11, 1167. |
| [10] | (c) Wang, C. Nat. Catal. 2018, 1, 816. |
| [10] | (d) Hu, Y.; Zhou, B.; Chen, H.; Wang, C. Angew. Chem., nt. Ed. 2018, 57, 12071. |
| [10] | (e) Zhou, B.; Hu, Y.; Liu, T.; Wang, C. Nat. Commun. 2017, 8, 1169. |
| [10] | (f) Hu, Y.; Wang, C. Sci. China Chem. 2016, 59, 1301. |
| [10] | (g) Zhou, B.; Hu, Y.; Wang, C. Angew. Chem., nt. Ed. 2015, 54, 13659. |
| [10] | (h) He, R.; Huang, Z.; Zheng, Q.; Wang, C. Angew. Chem., nt. Ed. 2014, 53, 4950. |
| [10] | (i) He, R.; Jin, X.; Chen, H.; Huang, Z.-T.; Zheng, Q.-Y.; Wang, C. J. Am. Chem. Soc. 2014, 136, 6558. |
| [10] | (j) Zhou, B.; Chen, H.; Wang, C. J. Am. Chem. Soc. 2013, 135, 1264. |
| [10] | (k) Wang, Z.; Chen, L.; Mao, G.; Wang, C. Chin. Chem. Lett. 2020, 31, 1890. |
| [10] | (l) Liang, Y.-F.; Steinbock, R.; Münch, A.; Stalke, D.; Ackermann, L. Angew. Chem., nt. Ed. 2018, 57, 5384. |
| [10] | (m) Wang, H.; Lorion, M. M.; Ackermann, L. Angew. Chem., nt. Ed. 2017, 56, 6339. |
| [10] | (n) Zhu, C.; Pinkert, T.; Greßies, S.; Glorius, F. ACS Catal. 2018, 8, 10036. |
| [10] | (o) Lu, Q.; Mondal, S.; Cembellín, S.; Glorius, F. Angew. Chem., nt. Ed. 2018, 57, 10732. |
| [10] | (p) Lu, Q.; Cembellín, S.; Greßies, S.; Singha, S.; Daniliuc, C. G.; Glorius, F. Angew. Chem., nt. Ed. 2018, 57, 1399. |
| [10] | (q) Hammarback, L. A.; Robinson, A.; Lynam, J. M.; Fairlamb, I. J. S. J. Am. Chem. Soc. 2019, 141, 2316. |
| [10] | (r) Zhou, X.; Li, Z.; Zhang, Z.; Lu, P.; Wang, Y. Org. Lett. 2018, 20, 1426. |
| [10] | (s) Liu, S.-L.; Li, Y.; Guo, J.-R.; Yang, G.-C.; Li, X.-H.; Gong, J.-F.; Song, M.-P. Org. Lett. 2017, 19, 4042. |
| [10] | (t) Ni, J.; Zhao, H.; Zhang, A. Org. Lett. 2017, 19, 3159. |
| [10] | (u) Chen, S.-Y.; Han, X.-L.; Wu, J.-Q.; Li, Q.; Chen, Y.; Wang, H. Angew. Chem., nt. Ed. 2017, 56, 9939. |
| [10] | (v) Pang, Y.; Liu, G.; Huang, C.; Yuan, X.; Li, W.; Xie, J. Angew. Chem., nt. Ed. 2020, 59, 12789. |
| [10] | (w) Dong, J.; Yuan, X.; Yan, Z.; Mu, L.; Ma, J.; Zhu, C.; Xie, J. Nat. Chem. 2021, 13, 182. |
| [10] | (x) Wang, D.; He, Y.; Dai, H.; Huang, C.; Yuan, X.; Xie, J. Chin. J. Chem. 2020, 38, 1497. |
| [11] | Lu, Q.; Klauck, F. J. R.; Glorius, F. Chem. Sci. 2017, 8, 3379. |
| [12] | (a) Lee, J. B.; Kang, M. E.; Kim, J.; Lee, C. Y.; Kee, J.-M.; Myung, K.; Park, J.-U.; Hong, S. Y. Chem. Commun. 2017, 53, 10394. |
| [12] | (b) Feng, Y.; Wu, Z.; Chen, T.; Fu, Q.; You, Q.; Shen, J.; Cui X. Chin. Chem. Lett. 2020, 31, 3263. |
| [12] | (c) Yu, Y.; Yue, Y.; Wang, D.; Li, X.; Chen, C.; Peng, J. Synthesis 2016, 48, 3941. |
| [12] | (d) Ghosh, P.; Ganguly, B.; Das, S. Org. Biomol. Chem. 2020, 18, 4497. |
| [13] | Jiang, X.; Yang, Q.; Yuan, J.; Deng, Z.; Mao, X.; Peng, Y.; Yu, C. Tetrahedron. 2016, 72, 1238. |
| [14] | Zheng, Y.; Song, W.-B.; Zhang, S.-W.; Xuan, L.-J. Org. Biomol. Chem. 2015, 13, 6474. |
| [15] | Kumaran, S.; Parthasarathy, K. Eur. J. Org. Chem. 2020, 866. |
| [16] | (a) Feng, Y.; Li, Y.; Yu, Y.; Wang, L.; Cui, X. RSC Adv. 2018, 8, 8450. |
| [16] | (b) Feng, Y.; Zhang, Z.; Fu, Q.; Yao, Q.; Huang, H.; Shen, J.; Cui, X. Chin. Chem. Lett. 2020, 31, 58. |
/
| 〈 |
|
〉 |