

胆碱酯酶抑制剂萘酰亚胺衍生物的合成与聚集诱导发光性质
收稿日期: 2021-07-30
修回日期: 2021-10-12
网络出版日期: 2021-11-17
基金资助
国家自然科学基金(U1704176); 河南省科技计划(212102311030); 河南应用技术职业学院(2020-HJ-22)
Synthesis of Naphthalimide Derivatives as Cholinesterase Inhibitors with Aggregation Induced Emission Properties
Received date: 2021-07-30
Revised date: 2021-10-12
Online published: 2021-11-17
Supported by
National Natural Science Foundation of China(U1704176); Science and Technology Planning Project of Henan Province(212102311030); Program of Henan Vocational College of Applied Technology(2020-HJ-22)
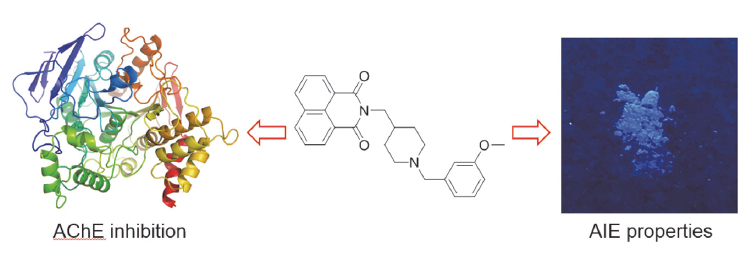
阿尔茨海默症(Alzheimer’s disease, AD)是一种神经退行性疾病, 严重影响老年人的生活质量, 目前治疗AD的药物主要是胆碱酯酶抑制剂, 如多奈哌齐、卡巴拉汀等. 本文基于多奈哌齐结构, 设计合成了一系列新的萘酰亚胺衍生物并进行了活性评价. 结果表明, 所合成的化合物均对乙酰胆碱酯酶(AChE)有选择性抑制, 其中2-((1-(3-甲氧基苄基)哌啶-4-基)甲基)-1H-苯并异喹啉-1,3(2H)-二酮(4k)的抑制活性最强, IC50值为4.43 μmol•L–1, 优于对照药物卡巴拉汀. 酶动力学及分子对接表明4k能够同时作用于AChE的催化活性位点和外周结合位点, 并且4k对SH-SY5Y和PC12细胞毒性较低. 此外, 这些化合物均显示出典型的聚集诱导发光(AIE)性质, 可能与萘酰亚胺分子内旋转受阻机制有关.
赵永梅 , 穆叶舒 , 罗稳 , 田智勇 . 胆碱酯酶抑制剂萘酰亚胺衍生物的合成与聚集诱导发光性质[J]. 有机化学, 2022 , 42(3) : 819 -829 . DOI: 10.6023/cjoc202107064
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by impaired cognitive and memory function, which seriously affects the quality of life of the elderly. At present, the main drugs for the treatment of AD are cholinesterase inhibitors, such as donepezil and rivastigmine. In this paper, a series of novel naphthimide derivatives were designed, synthesized and evaluated based on the structure of donepezil. The results showed that all of the tested compounds were acetylcholinesterase (AChE) selective inhibitors, 2-((1-(3-methoxybenzyl)piperidin-4-yl)methyl)-1H-benzo[de]isoquinoline- 1,3(2H)-dione (4k) exhibited the strongest inhibition to AChE with an IC50 value of 4.43 μmol/L, which was better than the control rivastigmine. Kinetic and molecular modeling studies indicated that 4k targeted both the catalytic active site (CAS) and the peripheral anionic site (PAS) of AChE. In addition, 4k didn’t show obvious cytotoxicity against SH-SY5Y and PC12 cell lines. Besides, these compounds exhibited typical aggregation induced emissions (AIE) properties, which might be controlled by mechanism of restriction of intramolecular rotations.

Key words: naphthalimide; cholinesterase; aggregation induced emission
| [1] | https://finance.sina.com.cn/china/gncj/2021-05-11/doc-ikmxzfmm1763811.shtml. |
| [2] | Scarpini, E.; Scheltens, P.; Feldman, H. Lancet Neurol. 2003, 2, 539. |
| [3] | Mimica, N.; Presečki, P. Psychiatr. Danub. 2009, 21, 108. |
| [4] | Du, C.-Q.; Xie, B.-H.; He, M.; Hu, Z.-Y.; Liu, Y.; He, X.; Liu, F.-Y.; Cheng, C.; Zhou, H.-B.; Huang, S.-T.; Dong, C.-E. Chin. J. Org. Chem. 2020, 40, 2035. (in Chinese) |
| [4] | (杜川黔, 谢宝花, 贺明, 胡志烨, 刘豫, 何雪, 刘凡玉, 程晨, 周海兵, 黄胜堂, 董春娥, 有机化学, 2020, 40, 2035.) |
| [5] | Zheng, S.-Y.; Yu, C.-H.; Shen, Z.-W. Chin. Chin. J. Org. Chem. 2013, 33, 2261. (in Chinese) |
| [5] | (郑书岩, 郁春辉, 沈征武, 有机化学, 2013, 33, 2261.) |
| [6] | Bortolami, M.; Rocco, D.; Messore, A.; Di Santo, R.; Costi, R.; Madia, V. N.; Scipione, L.; Pandolfi, F. Expert Opin. Ther. Pat. 2021, 31, 399. |
| [7] | Sang, Z.; Qiang, X.; Li, Y.; Xu, R.; Cao, Z.; Song, Q.; Wang, T.; Zhang, X.; Liu, H.; Tan, Z.; Deng, Y. Eur. J. Med. Chem. 2017, 135, 307. |
| [8] | Xu, Z.; Xiao, Y.; Qian, X.; Cui, J.; Cui, D. Org. Lett. 2005, 7, 889. |
| [9] | Duke, R. M.; Veale, E. B.; Pfeffer, F. M.; Kruger, P. E.; Gunnlaugsson, T. Chem. Soc. Rev. 2010, 39, 3936. |
| [10] | Xie, Z.-D.; Fu, M.-L.; Yin, B.; Zhu, Q. Chin. J. Org. Chem. 2018, 38, 1364. (in Chinese) |
| [10] | (谢振达, 付曼琳, 尹彪, 朱勍, 有机化学, 2018, 38, 1364.) |
| [11] | Tomczyk, M. D.; Walczak, K. Z. Eur. J. Med. Chem. 2018, 159, 393. |
| [12] | Stone, R. M.; Mazzola, E.; Neuberg, D.; Allen, S. L.; Pigneux, A.; Stuart, R. K.; Wetzler, M.; Rizzieri, D.; Erba, H. P.; Damon, L.; Jang, J. H.; Tallman, M. S.; Warzocha, K.; Masszi, T.; Sekeres, M. A.; Egyed, M.; Horst, H. A.; Selleslag, D.; Solomon, S. R.; Venugopal, P.; Lundberg, A. S.; Powell, B. J. Clin. Oncol. 2015, 33, 1252. |
| [13] | Chen, Z.; Xu, Y.; Qian, X. Chin. Chem. Lett. 2018, 29, 1741. |
| [14] | Tandon, R.; Luxami, V.; Kaur, H.; Tandon, N.; Paul, K. Chem. Rec. 2017, 17, 956. |
| [15] | Rong, R.-X.; Li, J.-M.; Li, Y.-F.; Guo, X.-Y.; Wang, C.; Li, Y.-J.; Li, J.-M.; Han, B.-J.; Cao, Z.-R.; Wang, K.-R.; Li, X.-L. Chin. J. Org. Chem. 2021, 41, 1599. (in Chinese) |
| [15] | (戎瑞雪, 李基民, 李耀文, 郭啸宇, 王冲, 李艳军, 李金梅, 韩宝君, 曹志然, 王克让, 李小六, 有机化学, 2021, 41, 1599.) |
| [16] | Luo, J.; Xie, Z.; Lam, J. W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 18, 1740. |
| [17] | Gopikrishna, P.; Meher, N.; Iyer, P. K. ACS Appl. Mater. Interfaces 2018, 10, 12081. |
| [18] | Ma, Y.; Zhao, L.; Li, Y.; Liu, J.; Yang, Y.; Chu, T. Tetrahedron 2018, 74, 2684. |
| [19] | Srivastava, A. K.; Singh, A. K.; Kumari, N.; Yadav, R.; Gulino, A.; Speghini, A.; Nagarajan, R.; Mishra, L. J. Lumin. 2017, 182, 274. |
| [20] | Ma, X.; Chi, W.; Han, X.; Wang, C.; Liu, S.; Liu, X.; Yin, J. Chin. Chem. Lett. 2021, 32, 1790. |
| [21] | Gao, J.; Midde, N.; Zhu, J.; Terry, A. V.; McInnes, C.; Chapman, J. M. Bioorg. Med. Chem. Lett. 2016, 26, 5573. |
| [22] | Blaikie, L.; Kay, G.; Kong Thoo Lin, P. Bioorg. Med. Chem. Lett. 2020, 30, 127505. |
| [23] | Gao, J.; Suo, C.; Tseng, J. H.; Moss, M. A.; Terry, A. V., Jr.; Chapman, J. Int. J. Mol. Sci. 2021, 22, 3120. |
| [24] | Dai, F.; Li, Q.; Wang, Y.; Ge, C.; Feng, C.; Xie, S.; He, H.; Xu, X.; Wang, C. J. Med. Chem. 2017, 60, 2071. |
| [25] | Li, M.; Wang, Y.; Ge, C.; Chang, L.; Wang, C.; Tian, Z.; Wang, S.; Dai, F.; Zhao, L.; Xie, S. Eur. J. Med. Chem. 2018, 143, 1732. |
| [26] | Alonso, D.; Dorronsoro, I.; Rubio, L.; Muñoz, P.; García-Palomero, E.; Monte, M. D.; Bidon-Chanal, A.; Orozco, M.; Luque, F. J.; Castro, A.; Medina, M.; Martínez, A. Bioorg. Med. Chem. 2005, 13, 6588. |
| [27] | Ellman, G. L.; Courtney, K. D.; Andres Jr, V.; Feather-Stone, R. M. Biochem. Pharmacol. 1961, 7, 88. |
| [28] | Elsinghorst, P. W.; Tanarro, C. M.; Gutschow, M. J. Med. Chem. 2006, 49, 7540. |
| [29] | Mao, F.; Chen, J.; Zhou, Q.; Luo, Z.; Huang, L.; Li, X. Bioorg. Med. Chem. Lett. 2013, 23, 6737. |
| [30] | Sukumaran, S. D.; Nasir, S. B.; Tee, J. T.; Buckle, M. J. C.; Othman, R.; Rahman, N. A.; Lee, V. S.; Bukhari, S. N. A.; Chee, C. F. J. Enzyme Inhib. Med. Chem. 2021, 36, 130. |
/
| 〈 |
|
〉 |