

中国南海矮小短指软珊瑚(Sinularia nanolobata)的化学成分研究
收稿日期: 2021-09-01
修回日期: 2021-09-27
网络出版日期: 2021-11-17
基金资助
国家重点研发计划(2018YFC0310903); 国家自然科学基金(81991521); 中国科学院上海药物研究所新药研究国家重点实验室项目(SIMM2103ZZ-06); 浙江省“万人计划”科技创新领军人才(2019R52009)
Chemical Constituents of Sinularia nanolobata from the South China Sea
Received date: 2021-09-01
Revised date: 2021-09-27
Online published: 2021-11-17
Supported by
National Key Research and Development Program(2018YFC0310903); National Natural Science Foundation of China(81991521); Project of State Key Laboratory of Drug Research/Shanghai Institute of Materia Medica(SIMM2103ZZ-06); Zhejiang Province “Ten Thousand Talents Program” Science and Technology Innovation Leading Talent Project(2019R52009)
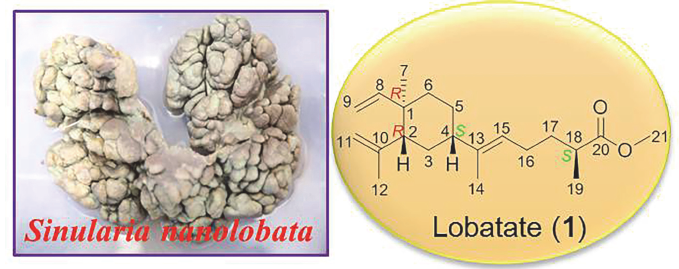
运用硅胶柱色谱、凝胶柱色谱和高效液相色谱等分离技术, 对中国南海西瑁岛矮小短指软珊瑚的丙酮提取物中的化学成分进行了系统研究, 从中分离得到10个化合物, 并采用多种波谱技术确定了这些化合物的化学结构. 分别鉴定为罗伯塔特(1)、(17R)-loba-8,10,13(15)-triene-17,18-diol (2)、α-生育醌(3)、(1E,3Z,7E,11R,12R)-11-羟基-12-甲氧基-1-异丙基-4,8,12-三甲基-环十四碳-1,3,7-三烯(4)、(+)-(1E,3E,7E,11R,12S)-11,12-epoxy-11,12-dihydrocembrene-C (5)、(1E,3E,7R,8S,11E)-7,8-环氧-1-异丙基-4,8,12-三甲基-环十四碳-1,3,11-三烯(6)、24-亚甲基-胆固醇(7)、孕烯醇酮(8)、3β,6α,11-三羟基-24-亚甲基-9,11-断-5α-胆甾-7-烯-9-酮(9), Sinugrandisterol A (10). 其中化合物1为新的异戊烯基榄烷型二萜化合物. 生物活性实验结果表明, 化合物3对蛋白酪氨酸磷酸酶1B (PTP1B)的酶活力具有较好的抑制活性, 其IC50为(4.83±2.35) μmol/L, 提示该化合物在治疗糖尿病和肥胖症的药物开发中具有一定的潜力.
曾子溶 , 张梦梦 , 王鸿 , 李佳 , 郭跃伟 , 苏明智 . 中国南海矮小短指软珊瑚(Sinularia nanolobata)的化学成分研究[J]. 有机化学, 2022 , 42(3) : 891 -895 . DOI: 10.6023/cjoc202109004
A new prenylelemane-type diterpenoid named lobatate (1), together with nine known compounds, namely (17R)- loba-8,10,13(15)-triene-17,18-diol (2), α-tocopherolquinone (3), (1E,3Z,7E,11R,12R)-11-hydroxy-12-methoxy-1-isopropyl- 4,8,12-trimethyl-cyclotetradeca-1,3,7-triene (4), (+)-(1E,3E,7E,11R,12S)-11,12-epoxy-11,12-dihydrocembrene-C (5), (1E,3E, 7R,8S,11E)-7,8-epoxy-1-isopropyl-4,8,12-trimethyl-cyclotetradeca-1,3,11-triene (6), 24-methylene-cholesterol (7), pregnenolone (8), 3β,6α,11-trihydroxy-24-methylene-9,11-seco-5α-cholest-7-en-9-one (9), sinugrandisterol A (10), was isolated from the soft coral Sinularia nanolobata, which were collected from the Ximao Island, Sanya, South China Sea. Their structures were elucidated using comprehensive spectroscopic methods. The biological activity experiment result showed that compound 3 significantly inhibited the enzymatic activity of protein tyrosine phosphatase-1B (PTP1B) with the IC50 value of (4.83± 2.35) μmol/L. The result indicated that compound 3 has a promising potential on the drug discovery of anti-diabetes and anti- obesity.

| [1] | Wang, Z.; Tang, X.-L.; Li, L.; Li, P.-L.; Li, G.-Q. Chin. J. Mar. Drugs 2018, 37, 47. (in Chinese) |
| [1] | 王正, 唐旭利, 李磊, 李平林, 李国强, 中国海洋药物, 2018, 37, 47). |
| [2] | Liang, L.-F.; Li, Y.-F.; Zheng, T.-T.; Yao, L.-G.; Guo, Y.-W. Chin. Trad. Herb Drugs 2017, 48, 868. (in Chinese) |
| [2] | (梁林富, 李玉芬, 郑亭亭, 姚励功, 郭跃伟, 中草药, 2017, 48, 868.) |
| [3] | Zou, X.; Li, P.-L.; Liu, B.-S.; Tang, X.-L.; Li, G.-Q. Chin. J. Mar. Drugs 2015, 34, 83. (in Chinese) |
| [3] | (邹雪, 李平林, 刘宝生, 唐旭利, 李国强, 中国海洋药物, 2015, 34, 83.) |
| [4] | Lu, S.-Q. M.S. Thesis, Jinzhou Medical University, Jinzhou, 2020. (in Chinese) |
| [4] | (卢思祺, 硕士论文, 锦州医科大学, 锦州, 2020.) |
| [5] | Ahmed, A. F.; Teng, W.-T.; Huang, C.-Y.; Dai, C.-F.; Hwang, T.-L.; Sheu, J.-H. Mar. Drugs 2017, 15, 300. |
| [6] | Ye, F.; Chen, Z.-H.; Gu, Y.-C.; Guo, Y.-W.; Li, X.-W. Chin. J. Nat. Med. 2020, 18, 839. |
| [7] | Nandi, S.; Saxena, M. Curr. Top. Med. Chem. 2020, 20, 2692. |
| [8] | Edrada, R. A.; Proksch, P.; Wray, V.; Witte, L.; Ofwegen, L. V. J. Nat. Prod. 1998, 61, 358. |
| [9] | Bonnard, I.; Jhaumeer-Laulloo, S. B.; Bontemps, N.; Banaigs, B.; Aknin, M. Mar. Drugs 2010, 8, 359. |
| [10] | Collado, I. G.; Hanson, J. R.; Macia-SanChez, A. J. Tetrahedron 1996, 52, 7961. |
| [11] | Jiang, C.-S.; Ru, T.; Yao, L.-G.; Miao, Z.-H.; Guo, Y.-W. Fitoterapia 2019, 136, 104176. |
| [12] | Musharraf, S. G.; Najeeb, A.; Khan, S.; Pervez, M.; Ali, R. A.; Choudhary, M. I. J. Mol. Catal. B: Enzym. 2010, 66, 156. |
| [13] | Qi, W.-Y.; Shen, Y.; Wu, Y.; Leng, Y.; Gao, K.; Yue, J.-M. Phytochemistry 2017, 136, 101. |
| [14] | Mi, J.-L.; Wu, C.-J.; Sun, L.-G.; Li, C.-T.; Xi, F.-M.; Chen, W.-S. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 86. (in Chinese) |
| [14] | (米君令, 吴纯洁, 孙灵根, 黎春彤, 习峰敏, 陈万生, 中国实验方剂学杂志, 2013, 19, 86.) |
| [15] | Liu, Z.; Li, W. Z.; Peng, L.; Li, Y.; Li, Y. J. Chem. Soc., erkin Trans. 1 2000, 24, 4250. |
| [16] | Lu, W.-G.; Zhang, C.-X.; Zeng, L.-M.; Su, J.-Y. Steroids 2004, 69, 803. |
| [17] | Yang, H.; Xie, J.-L.; Sun, H.-D. Acta Bot. Yunnanica 1997, 19, 92. (in Chinese) |
| [17] | (杨辉, 谢金伦, 孙汉董, 云南植物研究, 1997, 19, 92.) |
| [18] | Migliuolo, A.; Piccialli, V.; Sica, D. Steroids 1992, 57, 344. |
| [19] | Ahmed, A. F.; Tai, S.-H.; Wu, Y.-C.; Sheu, J.-H. Steroids 2007, 72, 368. |
/
| 〈 |
|
〉 |