

四乙烯五胺功能化酚醛树脂作为Knoevenagel缩合反应的高活性酸碱双功能催化剂
收稿日期: 2021-09-21
修回日期: 2021-11-06
网络出版日期: 2021-12-08
基金资助
河北省自然科学基金(H2020209288)
Tetraethylenepentamine Functionalized Phenolic Resin as Highly Active Acid-Base Bifunctional Catalyst for Knoevenagel Condensation Reaction
Received date: 2021-09-21
Revised date: 2021-11-06
Online published: 2021-12-08
Supported by
Natural Science Foundation of Hebei Province(H2020209288)
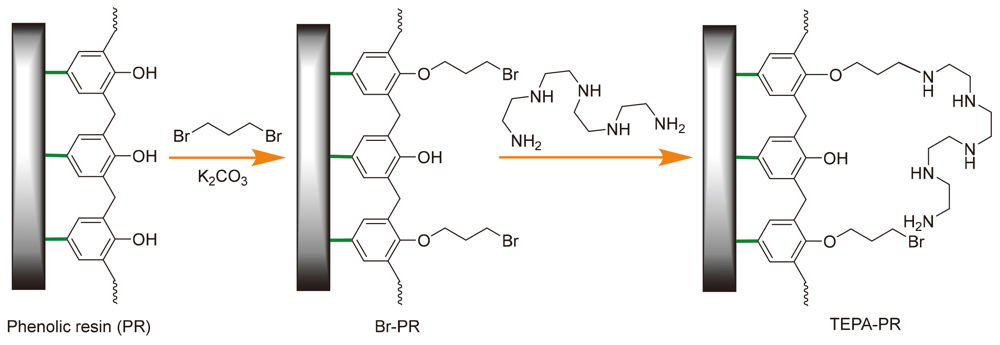
通过市售酚醛树脂(PR)和四乙烯五胺经由氨化反应制备四乙烯五胺官能化酚醛树脂(TEPA-PR), 并应用于催化Knoevenagel缩合反应. 通过傅里叶转移红外光谱(FTIR)、X射线衍射光谱(XRD)和元素分析(EA)表征TEPA-PR结构. TEPA-PR可高效催化芳香醛的Knoevenagel缩合反应, 具有产率高, 底物适用性广的优势. 此外, 该反应可在不同极性溶剂中进行. 该新型树脂催化剂表现出良好的可循环使用性, 使用5次后其催化活性没有显着降低.
关键词: 酚醛树脂; 酸碱催化; 多相催化; Knoevenagel缩合反应; 水相催化
肖剑 , 武志英 , 陈姿依 , 赵朋飞 , 刘春艳 . 四乙烯五胺功能化酚醛树脂作为Knoevenagel缩合反应的高活性酸碱双功能催化剂[J]. 有机化学, 2022 , 42(4) : 1179 -1187 . DOI: 10.6023/cjoc202109031
The tetraethylenepentamine functionalized phenolic resin (TEPA-PR) for Knoevenagel condensation reaction has been prepared by aminating a commercially available phenolic resin (PR) and tetraethylenepentamine. The TEPA-PR was characterized by Fourier-transfer infrared spectroscopy (FTIR), X-ray diffraction spectra (XRD) and elemental analysis (EA). The TEPA-PR can efficiently catalyze Knoevenagel condensation reaction of aromatic aldehydes with high yields of a wide range of products. Furthermore, the reaction can be easily carried out in different solvents of different polarity. The novel developed resin catalyst shows good reusability (5 times) without significant decrease of catalytic activity.

| [1] | (a) Varga G.; Kukovecz Á.; Kónya Z.; Sipos P.; Pálinkó I. J. Catal. 2020, 381, 308. |
| [1] | (b) Moteki T.; Koga Y.; Ogura M. J. Catal. 2019, 378, 131. |
| [1] | (c) Zacuto M. J. J. Org. Chem. 2019, 84, 6465. |
| [1] | (d) Li J. P. H.; Kennedy E. M.; Adesina A. A.; Stockenhuber M. J. Catal. 2019, 369, 157. |
| [1] | (e) Jia Y.; Fang Y.; Zhang Y.; Miras H. N.; Song Y. Chem.-Eur. J. 2015, 21, 14862. |
| [1] | (f) Garrabou X.; Wicky B. I. M.; Hilvert D. J. Am. Chem. Soc. 2016, 138, 6972. |
| [1] | (g) Franconetti A.; Domínguez-Rodríguez P.; Lara-García D.; Prado-Gotor R.; Cabrera-Escribano F. Appl. Catal. A: Gen. 2016, 517, 176. |
| [1] | (h) Ezugwu C. I.; Mousavi B.; Asraf M. A.; Luo Z.; Verpoort F. J. Catal. 2016, 344, 445. |
| [2] | (a) Kumar N. S.; Reddy M. S.; S. Kumar T. S.; Bheeram V. R.; Mukkamala S. B.; Rao L. C. ChemistrySelect 2019, 4, 1188. |
| [2] | (b) Gu X.; Tang Y.; Zhang X.; Luo Z.; Lu H. New J. Chem. 2016, 40, 6580. |
| [2] | (c) Pandey R.; Singh D.; Thakur N.; Raj K. K. ACS Omega 2021, 6, 13240. |
| [2] | (d) Grass J.; Klühspies K.; Reiprich B.; Schwieger W.; Inayat A. Catalysts 2021, 11, 474. |
| [2] | (e) Das A.; Anbu N.; SK M. Dalton Trans. 2019, 4, 17371. |
| [2] | (f) Zhang X.; Zhang R.; Jin Y.; Li T. J. Solid State Chem. 2019, 278, 120927. |
| [2] | (g) Guo C. Y.; Zhang Y. H.; Zhang L.; Zhang Y.; Wang J. D. CrystEngComm 2018, 20, 5327. |
| [3] | (a) Das A.; Anbu N.; Dhakshinamoorthy A.; Biswas S. Microporous Mesoporous Mater. 2019, 284, 459. |
| [3] | (b) Gao M.; Qi M.; Liu L.; Han Z. Chem. Commun. 2019, 55, 6377. |
| [4] | (a) Zhang X. Y.; He X. Y.; Zhao S. H. Green Chem Lett. Rev. 2021, 14, 85. |
| [4] | (b) Jiang Y.; Jiang F.; Liao X.; Lai S.; Wang S.; Xiong X.; Zheng J.; Liu Y. J. Porous Mater. 2020, 27, 779. |
| [5] | (a) Appaturi J. N.; Pulingam T.; Rajabathar J. R.; Khoerunnisa F.; Ling T. C.; Tan S. H.; Ng E. Microporous Mesoporous Mater. 2021, 320, 111091. |
| [5] | (b) Li R. J.; Chen C.; Hu L. Y.; ChemistrySelect 2020, 5, 14578. |
| [6] | (a) Jaenicke S.; Chuah G. K.; Lin X. H.; Hu X. C. Micro¬porous Mesoporous Mater. 2000, 35-36, 143. |
| [7] | (a) Qian B.; Wang F.; Li D.; Li Y. Zhang B.; Zhu J. New J. Chem. 2020, 44, 5995. |
| [7] | (b) Zhu J.; Wang F.; Li D.; Zhai J.; Liu P.; Zhang W.; Li Y.; Catal. Lett. 2020, 150, 1909. |
| [7] | (c) Xue B.; Liu X.; Liu N.; Li Y. Res. Chem. Intermed. 2018, 44, 1523. |
| [8] | (a) Hajizadeh F.; Maleki B.; Zonoz F. M.; Amiri A. J. Iran. Chem. Soc. 2021, 18, 793. |
| [8] | (b) Hu Y.; Zhang J.; Wang Z.; Huo H.; Jiang Y.; Xu X.; Lin K. ACS Appl. Mater. Inter. 2020, 12, 36159. |
| [9] | (a) Zhang X.; He X.; Zhao S. Green Chem. Lett. Rev. 2021, 14, 85. |
| [9] | (b) Gilanizadeh M.; Zeynizadeh B. Can. J. Chem. 2021, 99, 531. |
| [10] | (a) Yao C.; Zhou S.; Kang X.; Zhao Y.; Yan R.; Zhang Y.; Wen L. Inorg. Chem. 2018, 57, 11157. |
| [10] | (b) Sarmah B.; Srivastava R. Mol. Catal. 2017, 427, 62. |
| [10] | (c) Zhang L.; Wang H.; Shen W.; Qin Z.; Wang J.; Fan W. J. Catal. 2016, 344, 293. |
| [11] | Xiao J.; Wang L.; Ran J.; Zhao J.; Ma N.; Tao M.; Zhang W. J. Cleaner Prod. 2020, 274, 122473. |
| [12] | Laha B.; Khullar S.; Gogia A.; Mandal S. K. Dalton Trans. 2020, 49, 12298. |
| [13] | Sadjadi S.; Akbari M.; Kahangi F. G.; Heravi M. M. Polyhedron 2020, 179, 114375. |
| [14] | Tian H.; Liu S.; Zhang Z.; Dang T.; Lu Y.; Liu S. ACS Sustainable Chem. Eng. 2021, 9, 4660. |
| [15] | Zabihzadeh M.; Omidi A.; Shirini F.; Tajik H.; Langarudi M. S. N. J. Mol. Struct. 2020, 1206, 127730. |
| [16] | Wen H.; Xie J.; Zhou Y.; Zhou Y.; Wang J. Catal. Sci. Technol. 2019, 9, 5725. |
| [17] | Xu Q.; Niu Y.; Wang G.; Li Y.; Zhao Y.; Singh V.; Niu J.; Wang J. Mol. Catal. 2018, 453, 93. |
| [18] | Taher A.; Lee D.; Lee B.; Lee I. Synlett. 2016, 27, 1433. |
| [19] | Modak A.; Mondal J.; Bhaumik A. Appl. Catal. A: Gen. 2013, 459, 41. |
| [20] | Rong N.; Qiu T.; Qian R.; Lu L.; Huang X.; Ma Z.; Cui C. Inorg. Chem. Commun. 2017, 86, 98. |
| [21] | Blanco-Ania D.; Valderas-Cortina C.; Hermkens P. H. H.; Sliedregt L. A. J. M.; Scheeren H. W.; Rutjes F. P. J. T. Molecules 2010, 15, 2269. |
| [22] | Balalaie S.; Bararjanian M.; Hekmat S.; Salehi P. Synth. Commun. 2006, 36, 3703. |
| [23] | Xu H.; Pan L.; Fang X.; Liu B.; Zhang W.; Lu M.; Chang H. Tetrahedron Lett. 2017, 58, 2360. |
| [24] | Li J.; Lear M. J.; Hayashi Y. Chem.-Eur. J. 2021, 27, 5901. |
| [25] | Shi D. Q.; Chen J.; Zhuang Q. Y.; Wang X. S.; Hu H. W. Chin. Chem. Lett. 2003, 14, 1242. |
| [26] | Tukhtaev H. B.; Ivanov K. L.; Bezzubov S. I.; Cheshkov D. A.; Melnikov M. Y.; Budynina E. M. Org. Lett. 2019, 21, 1087. |
| [27] | Ren Y. M.; Cai C. Catal. Lett. 2007, 118, 134. |
| [28] | Wiles C.; Watts P. Haswell S. J. Lab Chip 2007, 7, 322. |
| [29] | Yadav J. S.; Reddy B. V. S.; Basak A. K.; Visali B.; Narsaiah A. V.; Nagaiah K. Eur. J. Org. Chem. 2004, 2004, 546. |
/
| 〈 |
|
〉 |