

可见光诱导α-氨基酸衍生物脱羧偶联反应研究进展
收稿日期: 2021-10-14
修回日期: 2021-11-25
网络出版日期: 2021-12-15
基金资助
国家自然科学基金(21602027); 国家自然科学基金(11765002); 江西省自然科学基金重点(2021ACB203001)
Recent Advances in Visible-Light-Induced Decarboxylative Coupling Reactions of α-Amino Acid Derivatives
Received date: 2021-10-14
Revised date: 2021-11-25
Online published: 2021-12-15
Supported by
National Natural Science Foundation of China(21602027); National Natural Science Foundation of China(11765002); Key Project of Natural Science Foundation of Jiangxi Province(2021ACB203001)
胡家榆 , 祝志强 , 谢宗波 , 乐长高 . 可见光诱导α-氨基酸衍生物脱羧偶联反应研究进展[J]. 有机化学, 2022 , 42(4) : 978 -1001 . DOI: 10.6023/cjoc202110020
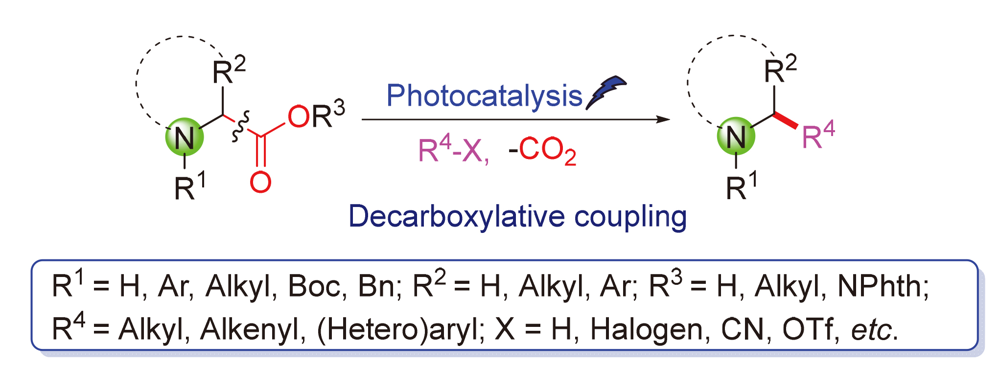
Amino acids are an important class of biomass raw materials, which are widely used as starting materials to synthetize bioactive molecules, drugs and functional materials, and are also useful as organic catalysts or metal ligands for asymmetric synthesis. Visible-light-induced organic transformation has attracted enormous interest due to its intrinsic characteristics of sustainability and green chemistry. The use of visible-light to promote decarboxylative coupling of α-amino acids to construct various nitrogen-containing compounds has been considered as an attractive synthetic strategy. This review highlights the recent progress in photocatalytic decarboxylative coupling reactions of α-amino acid derivatives with various partners.
| [1] | (a) Pollegioni, L.; Servi, S. Non-natural Amino Acids: Methods and Protocols, Springer, New York, 2012. |
| [1] | (b) Hughes, A. B. Amino Acids, Peptides and Proteins in Organic Chemistry, Wiley VCH, Weinheim, 2011. |
| [1] | (c) Li, B.; Liu, R.; Liang, R.; Jia, Y. Acta Chim. Sinica 2017, 75, 448. (in Chinese) |
| [1] | ( 李保乐, 刘人荣, 梁仁校, 贾义霞, 化学学报, 2017, 75, 448.) |
| [1] | (d) Mei, P.; Zhang, Y.; Feng, X. Acta Chim. Sinica 2020, 78, 1041. (in Chinese) |
| [1] | ( 梅佩, 张媛媛, 冯霄, 化学学报, 2020, 78, 1041.) |
| [2] | (a) He, G.; Wang, B.; Nack, W. A.; Chen, G. Acc. Chem. Res. 2016, 49, 635. |
| [2] | (b) Soloshonok, V. A.; Izawa, K.In Asymmetric Synthesis and Application of α-Amino Acids, American Chemical Society, Washington DC, 2009, Vol. 1009. |
| [3] | Chaloner, P. A. J. Organomet. Chem. 1987, 337, 431. |
| [4] | Li, J. L.; Shi, J.; Yu, Q. W.; Wang, W. Q.; Yang, J. M.; Lv, J. Fine Chem. 2019, 36, 1501. (in Chinese) |
| [4] | ( 李佳霖, 石坚, 余秦伟, 王为强, 杨建明, 吕剑, 精细化工, 2019, 36, 1501.) |
| [5] | (a) Wei, Y.; Hu, P.; Zhang, M.; Su, W. Chem. Rev. 2017, 117, 8864. |
| [5] | (b) Cornella, J.; Edwards, J. T.; Qin, T.; Kawamura, S.; Wang, J.; Pan, C.-M.; Gianatassio, R.; Schmidt, M.; Eastgate, M. D.; Baran, P. S. J. Am. Chem. Soc. 2016, 138, 2174. |
| [5] | (c) Jin, J.; Zhang, F.; Wang, Y. Acta Chim. Sinica 2019, 77, 889. (in Chinese) |
| [5] | ( 靳继康, 张凤莲, 汪义丰, 化学学报, 2019, 77, 889.) |
| [6] | Xuan, J.; Zhang, Z. G.; Xiao, W. J. Angew. Chem., Int. Ed. 2015, 54, 15632. |
| [7] | Jin, Y.; Fu, H. Asian J. Org. Chem. 2017, 6, 368. |
| [8] | Tong, Z.; Wang, N. X.; Xing, Y. J. Org. Chem. 2018, 83, 7559. |
| [9] | Guo, J.; Xie, Y.; Wu, Q.-L. RSC Adv. 2018, 8, 16202. |
| [10] | (a) Xie, J.; Jin, H.; Xu, P.; Zhu, C. Tetrahedron Lett. 2014, 55, 36. |
| [10] | (b) Chen, J.; Cen, J.; Xu, X.; Li, X. Catal. Sci. Technol. 2016, 6, 349. |
| [10] | (c) Yang, H.-Q.; Chen, Q.-Q.; Liu, F.; Shi, R.; Chen, Y. Chin. Chem. Lett. 2021, 32, 676. |
| [11] | Zuo, Z.; MacMillan, D. W. J. Am. Chem. Soc. 2014, 136, 5257. |
| [12] | Noble, A.; MacMillan, D. W. C. J. Am. Chem. Soc. 2014, 136, 11602. |
| [13] | Chu, L.; Ohta, C.; Zuo, Z.; MacMillan, D. W. J. Am. Chem. Soc. 2014, 136, 10886. |
| [14] | Inuki, S.; Sato, K.; Fukuyama, T.; Ryu, I.; Fujimoto, Y. J. Org. Chem. 2017, 82, 1248. |
| [15] | Vaillant, F. L.; Courant, T.; Waser, J. Angew. Chem., Int. Ed. 2015, 54, 11200. |
| [16] | Vaillant, F. L.; Wodrich, M. D.; Waser, J. Chem. Sci. 2017, 8, 1790. |
| [17] | McCarver, S. J.; Qiao, J. X.; Carpenter, J.; Borzilleri, R. M.; Poss, M. A.; Eastgate, M. D.; Miller, M. M.; MacMillan, D. W. Angew. Chem., nt. Ed. 2017, 56, 728. |
| [18] | Lovett, G. H.; Sparling, B. A. Org. Lett. 2016, 18, 3494. |
| [19] | Millet, A.; Lefebvre, Q.; Rueping, M. Chem. Eur. J. 2016, 22, 13464. |
| [20] | Jin, Y.; Yang, H.; Fu, H. Org. Lett. 2016, 18, 6400. |
| [21] | Li, J.; Lefebvre, Q.; Yang, H.; Zhao, Y.; Fu, H. Chem. Commun. 2017, 53, 10299. |
| [22] | Zhang, M.-J.; Schroeder, G. M.; He, Y.-H.; Guan, Z. RSC Adv. 2016, 6, 96693. |
| [23] | He, Y.-H.; Xiang, Y.; Yang, D.-C.; Guan, Z. Green Chem. 2016, 18, 5325. |
| [24] | Zhang, G.-Y.; Xiang, Y.; Guan, Z.; He, Y.-H. Catal. Sci. Technol. 2017, 7, 1937. |
| [25] | Gao, F.; Wang, J. T.; Liu, L. L.; Ma, N.; Yang, C.; Gao, Y.; Xia, W. Chem. Commun. 2017, 53, 8533. |
| [26] | Noble, A.; Mega, R. S.; Pflasterer, D.; Myers, E. L.; Aggarwal, V. K. Angew. Chem., Int. Ed. 2018, 57, 2155. |
| [27] | Shao, T.; Yin, Y.; Lee, R.; Zhao, X.; Chai, G.; Jiang, Z. Adv. Synth. Catal. 2018, 360, 1754. |
| [28] | Kolmel, D. K.; Loach, R. P.; Knauber, T.; Flanagan, M. E. ChemMedChem 2018, 13, 2159. |
| [29] | Bloom, S.; Liu, C.; Kolmel, D. K.; Qiao, J. X.; Zhang, Y.; Poss, M. A.; Ewing, W. R.; MacMillan, D. W. C. Nat. Chem. 2018, 10, 205. |
| [30] | Ji, J.-J.; Zhu, Z.-Q.; Xiao, L.-J.; Guo, D.; Zhu, X.; Tang, J.; Wu, J.; Xie, Z.-B.; Le, Z.-G. Org. Chem. Front. 2019, 6, 3693. |
| [31] | Zhou, Z.; Nie, X.; Harms, K.; Riedel, R.; Zhang, L.; Meggers, E. Sci. China Chem. 2019, 62, 1512. |
| [32] | Liu, X.; Yin, Y.; Jiang, Z. Chem. Commun. 2019, 55, 11527. |
| [33] | Yang, H.; Wei, G.; Jiang, Z. ACS Catal. 2019, 9, 9599. |
| [34] | Wang, Y. T.; Fu, M. C.; Zhao, B.; Shang, R.; Fu, Y. Chem. Commun. 2020, 56, 2495. |
| [35] | Shao, M.; Liang, H.; Liu, Y. L.; Qin, W.; Li, Z. Asian J. Org. Chem. 2020, 9, 782. |
| [36] | Si, Y.-F.; Chen, X.-L.; Fu, X.-Y.; Sun, K.; Song, X.; Qu, L.-B.; Yu, B. ACS Sustain. Chem. Eng. 2020. 8, 10740. |
| [37] | Si, Y. F.; Sun, K.; Chen, X. L.; Fu, X. Y.; Liu, Y.; Zeng, F. L.; Shi, T.; Qu, L. B.; Yu, B. Org. Lett. 2020, 22, 6960. |
| [38] | Gueret, R.; Pelinski, L.; Bousquet, T.; Sauthier, M.; Ferey, V.; Bigot, A. Org. Lett. 2020, 22, 5157. |
| [39] | Pan, S.; Jiang, M.; Zhong, G.; Dai, L.; Zhou, Y.; Wei, K.; Zeng, X. Org. Chem. Front. 2020, 7, 4043. |
| [40] | Pan, S.; Jiang, M.; Hu, J.; Xu, R.; Zeng, X.; Zhong, G. Green Chem. 2020, 22, 336. |
| [41] | Li, H. H.; Li, J. Q.; Zheng, X.; Huang, P. Q. Org. Lett. 2021, 23, 876. |
| [42] | Bao, Q. F.; Li, M.; Xia, Y.; Wang, Y. Z.; Zhou, Z. Z.; Liang, Y. M. Org. Lett. 2021, 23, 1107. |
| [43] | Liao, L.-L.; Cao, G.-M.; Jiang, Y.-X.; Jin, X.-H.; Hu, X.-L.; Chruma, J. J.; Sun, G.-Q.; Gui, Y.-Y.; Yu, D.-G. J. Am. Chem. Soc. 2021, 143, 2812. |
| [44] | Zhou, C.; Li, M.; Sun, J.; Cheng, J.; Sun, S. Org. Lett. 2021, 23, 2895. |
| [45] | Hu, W.; Zhan, Q.; Zhou, H.; Cao, S.; Jiang, Z. Chem. Sci. 2021, 12, 6543. |
| [46] | Yang, J.; Song, M.; Zhou, H.; Qi, Y.; Ma, B.; Wang, X.-C. Green Chem. 2021, 23, 5806. |
| [47] | Wang, J.-X.; Wang, Y.-T.; Zhang, H.; Fu, M.-C. Org. Chem. Front. 2021, 8, 4466. |
| [48] | Li, Y.; Dai, C.; Xie, S.; Liu, P.; Sun, P. Org. Lett. 2021, 23, 5906. |
| [49] | (a) Nicewicz, D. A.; Macmillan, D. Science 2008, 322, 77. |
| [49] | (b) Narayanam, J. M. R.; Tucker, J. W.; Stephenson, C. R. J. J. Am. Chem. Soc. 2009, 131, 8756. |
| [50] | (a) Shaw, M. H.; Shurtleff, V. W.; Terrett, J. A.; Cuthbertson, J. D.; Macmillan, D. W. C. Science 2016, 47, 1304. |
| [50] | (b) Zhang, H.-H.; Yu, S. Acta Chim. Sinica 2019, 77, 832. (in Chinese) |
| [50] | ( 张洪浩, 俞寿云, 化学学报, 2019, 77, 832.) |
| [51] | Zuo, Z.; Ahneman, D. T.; Chu, L.; Terrett, J. A.; Doyle, A. G.; MacMillan, D. W. C. Science 2014, 345, 437. |
| [52] | Oderinde, M. S.; Varela-Alvarez, A.; Aquila, B.; Robbins, D. W.; Johannes, J. W. J. Org. Chem. 2015, 80, 7642. |
| [53] | Zuo, Z.; Cong, H.; Li, W.; Choi, J.; Fu, G. C.; MacMillan, D. W. J. Am. Chem. Soc. 2016, 138, 1832. |
| [54] | Zhang, H.; Zhang, P.; Jiang, M.; Yang, H.; Fu, H. Org. Lett. 2017, 19, 1016. |
| [55] | Fan, L.; Jia, J.; Hou, H.; Lefebvre, Q.; Rueping, M. Chem. Eur. J. 2016, 22, 16437. |
| [56] | Cartwright, K. C.; Lang, S. B.; Tunge, J. A. J. Org. Chem. 2019, 84, 2933. |
| [57] | Cartwright, K. C.; Tunge, J. A. ACS Catal. 2018, 8, 11801. |
| [58] | Kolmel, D. K.; Meng, J.; Tsai, M. H.; Que, J.; Loach, R. P.; Knauber, T.; Wan, J.; Flanagan, M. E. ACS Comb. Sci. 2019, 21, 588. |
| [59] | Yue, H.; Zhu, C.; Kancherla, R.; Liu, F.; Rueping, M. Angew. Chem., Int. Ed. 2020, 59, 5738. |
| [60] | Zhang, Y.; Mao, L.-L.; Hu, S.; Luan, Y.; Cong, H. Chin. Chem. Lett. 2021, 32, 681. |
| [61] | Cheng, W.-M.; Shang, R.; Fu, Y. ACS Catal. 2017, 7, 907. |
| [62] | Fu, M.-C.; Shang, R.; Zhao, B.; Wang, B.; Fu, Y. Science 2019, 363, 1429. |
| [63] | Proctor, R. S. J.; Davis, H. J.; Phipps, R. J. Science 2018, 360, 419. |
| [64] | Liu, X.; Liu, Y.; Chai, G.; Qiao, B.; Zhao, X.; Jiang, Z. Org. Lett. 2018, 20, 6298. |
| [65] | Yin, Y.; Dai, Y.; Jia, H.; Li, J.; Bu, L.; Qiao, B.; Zhao, X.; Jiang, Z. J. Am. Chem. Soc. 2018, 140, 6083. |
| [66] | Liu, Y.; Liu, X.; Li, J.; Zhao, X.; Qiao, B.; Jiang, Z. Chem. Sci. 2018, 9, 8094. |
| [67] | Li, J.; Kong, M.; Qiao, B.; Lee, R.; Zhao, X.; Jiang, Z. Nat. Commun. 2018, 9, 2445. |
| [68] | Zeng, G.; Li, Y.; Qiao, B.; Zhao, X.; Jiang, Z. Chem. Commun. 2019, 55, 11362. |
| [69] | Zhang, Z.; Song, X.; Li, G.; Li, X.; Zheng, D.; Zhao, X.; Miao, H.; Zhang, G.; Liu, L. Chin. Chem. Lett. 2021, 32, 1423. |
| [70] | Li, J.; Gu, Z.; Zhao, X.; Qiao, B.; Jiang, Z. Chem. Commun. 2019, 55, 12916. |
| [71] | Shen, M. L.; Shen, Y.; Wang, P. S. Org. Lett. 2019, 21, 2993. |
/
| 〈 |
|
〉 |