

链霉菌CB03234-S中天赐霉素衍生物的发现
收稿日期: 2021-11-10
修回日期: 2021-12-09
网络出版日期: 2021-12-31
基金资助
国家自然科学基金(81872779); 天然药物及仿生药物国家重点实验室开放基金(K202116)
Discovery of Tiancimycin Congeners from Streptomyces sp. CB03234-S
Received date: 2021-11-10
Revised date: 2021-12-09
Online published: 2021-12-31
Supported by
National Science Foundation of China(81872779); State Key Laboratory of Natural and Biomimetic Drugs(K202116)
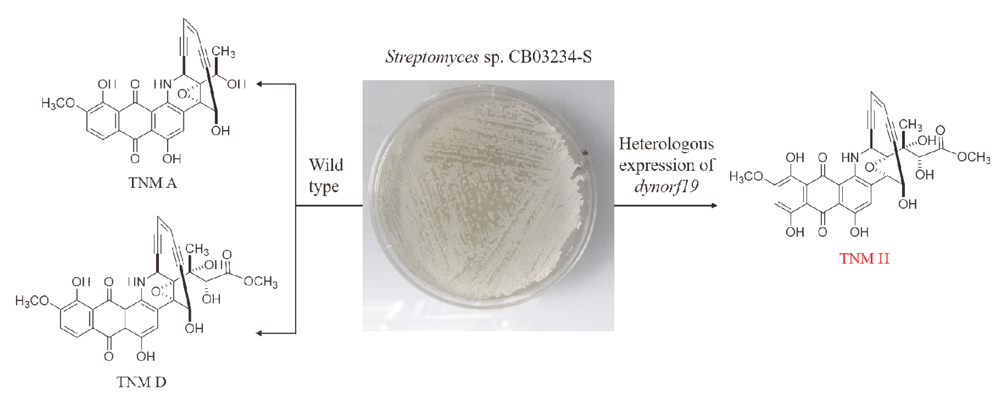
天赐霉素是一种蒽醌骈合型烯二炔类化合物, 具有显著的抗肿瘤活性. 通过在天赐霉素A高产菌株放线菌CB03234-S中异源表达两个细胞色素P450羟化酶基因dynE10和dynOrf19, 分离得到三个天赐霉素衍生物1~3. 通过一维和二维核磁共振、高分辨质谱和圆二色谱测定了它们的化学结构. 天赐霉素H (1)为天赐霉素D的C-9羟基化产物, TNM T1 (2)为一个烯二炔的环化产物, TNM T3 (3)为已知化合物. 化合物1对所有四种测试的肿瘤细胞株, 如HeLa细胞、MB49细胞、B16细胞和BIU87细胞均具有较强的抗肿瘤活性.
关键词: 天赐霉素; 蒽醌骈合型烯二炔; 细胞色素P450羟化酶; 抗肿瘤活性; 异源表达
薛露 , 张丽花 , 张成玉 , 赵鑫 , 党伟帆 , 王昭昕 , 王春华 , 所同川 , 颜晓晖 . 链霉菌CB03234-S中天赐霉素衍生物的发现[J]. 有机化学, 2022 , 42(4) : 1241 -1247 . DOI: 10.6023/cjoc202111018
Tiancimycins (TNMs) are anthraquinone-fused enediynes with phenomenal antitumor activity. By heterologous expression of two cytochrome P450 hydroxylase genes, dynE10 and dynOrf19, in the TNMs high-producing strain Streptomyces sp. CB03234-S, three TNM derivatives 1~3 were isolated. Their chemical structures were determined by one-dimensional and two-dimensional NMR, high resolution mass spectrometry and circular dichroism. TNM H (1) is the C-9-hydroxylated product of TNM D, TNM T1 (2) is a cycloaromatized product, and TNM T3 (3) is a known compound. Compound 1 exhibited strong antitumor activity against the four tested cancer cell lines, including HeLa, MB49, B16 and BIU87.

| [1] | Yan, X. Nat. Prod. Rep. 2021, 39, 703. |
| [2] | Yan, X.; Ge, H.; Huang, T.; Hindra; Yang, D.; Teng, Q.; Crnovčić, I.; Li, X.; Rudolf, J. D.; Lohman, J. R.; Gansemans, Y.; Zhu, X.; Huang, Y.; Zhao, L. X.; Jiang, Y.; Van Nieuwerburgh, F.; Rader, C.; Duan, Y.; Shen, B. mBio 2016, 7, e02104. |
| [3] | Konishi, M.; Ohkuma, H.; Matsumoto, K.; Tsuno, T.; Kamei, H.; Miyaki, T.; Oki, T.; Kawaguchi, H.; VanDuyne, G. D.; Clardy, J. J. Antibiot. (Tokyo) 1989, 42, 1449. |
| [4] | Davies, J.; Wang, H.; Taylor, T.; Warabi, K.; Huang, X. H.; Andersen, R. J. Org. Lett. 2005, 7, 5233. |
| [5] | Yan, X.; Chen, J. J.; Adhikari, A.; Yang, D.; Crnovcic, I.; Wang, N.; Chang, C. Y.; Rader, C.; Shen, B. Org. Lett. 2017, 19, 6192. |
| [6] | Igarashi, M.; Sawa, R.; Umekita, M.; Hatano, M.; Arisaka, R.; Hayashi, C.; Ishizaki, Y.; Suzuki, M.; Kato, C. J. Antibiot. (Tokyo) 2021, 74, 291. |
| [7] | Poudel, Y. B.; Rao, C.; Kotapati, S.; Deshpande, M.; Thevanayagam, L.; Pan, C.; Cardarelli, J.; Chowdari, N.; Kaspady, M.; Samikannu, R.; Kuppusamy, P.; Saravanakumar, P.; Arunachalam, P. N.; Deshpande, S.; Rangan, V.; Rampulla, R.; Mathur, A.; Vite, G. D.; Gangwar, S. Bioorg. Med. Chem. Lett. 2020, 30, 126782. |
| [8] | Nicolaou, K. C.; Rigol, S.; Pitsinos, E. N.; Das, D.; Lu, Y.; Rout, S.; Schammel, A. W.; Holte, D.; Lin, B.; Gu, C.; Sarvaiya, H.; Trinidad, J.; Barbour, N.; Valdiosera, A. M.; Sandoval, J.; Lee, C.; Aujay, M.; Fernando, H.; Dhar, A.; Karsunky, H.; Taylor, N.; Pysz, M.; Gavrilyuk, J. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2107042118. |
| [9] | Annaval, T.; Teijaro, C. N.; Adhikari, A.; Yan, X.; Chen, J. J.; Crnovcic, I.; Yang, D.; Shen, B. ACS. Chem. Biol. 2021, 16, 1172. |
| [10] | Cohen, D. R.; Townsend, C. A. Nat. Chem. 2018, 10, 231. |
| [11] | Gao, Q.; Thorson, J. S. FEMS Microbiol. Lett. 2008, 282, 105. |
| [12] | Yan, X.; Chen, J. J.; Adhikari, A.; Teijaro, C. N.; Ge, H.; Crnovcic, I.; Chang, C. Y.; Annaval, T.; Yang, D.; Rader, C.; Shen, B. Org. Lett. 2018, 20, 5918. |
| [13] | Zhuang, Z.; Jiang, C.; Zhang, F.; Huang, R.; Yi, L.; Huang, Y.; Yan, X.; Duan, Y.; Zhu, X. Biotechnol. Bioeng. 2019, 116, 1304. |
/
| 〈 |
|
〉 |