

一种利用酰胺基转移反应脱除酰基保护基的实用方法
收稿日期: 2021-12-03
修回日期: 2021-12-30
网络出版日期: 2022-01-11
基金资助
国家自然科学基金(21871019); 北京工商大学北京市食品风味化学重点实验室开放课题基金资助项目(BTBU)
A Practical Transamidation Strategy for the N-Deacylation of Amides
Received date: 2021-12-03
Revised date: 2021-12-30
Online published: 2022-01-11
Supported by
National Natural Science Foundation of China(21871019); Open Project Program of Beijing Key Laboratory of Flavor Chemistry, Beijing Technology and Business University(BTBU)
韩群 , 徐坤 , 田发宁 , 黄胜阳 , 曾程初 . 一种利用酰胺基转移反应脱除酰基保护基的实用方法[J]. 有机化学, 2022 , 42(4) : 1123 -1128 . DOI: 10.6023/cjoc202112007
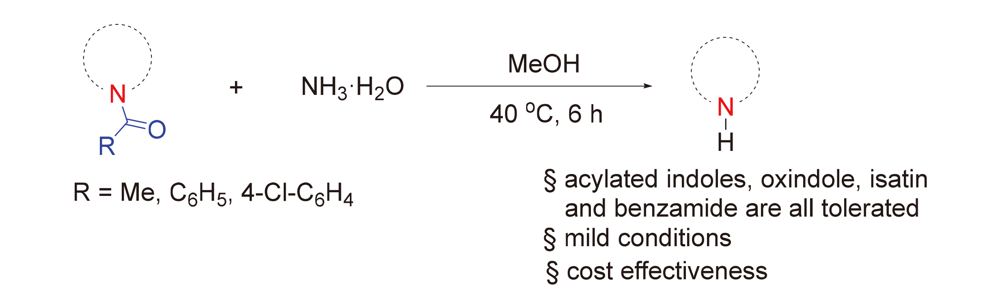
The N-deacylation of amides under mild conditions is highly desirable in organic synthesis, but it remains challenging due to the chemically robust nature of amide bond. A general solution to the N-deacylation of amides with NH3•H2O under mild and scalable conditions (10 mmol) was developed. A range of drugs and drug derivatives including indomethacin, N-acetyl melatonin and N-acetyl carprofen could be deacylated to release free amines in excellent yields. The good functional group compatibility, combined with operational simplicity, excellent yield and cost effectiveness of all reagents, makes this protocol a prime candidate for N-deacylation of amide.
Key words: deacylation; transamidation; green synthesis; amide
| [1] | (a) Wang, H.; Shi, J.; Tan, J.; Xu, W.; Zhang, S.; Xu, K. Org. Lett. 2019, 21, 9430. |
| [1] | (b) Wang, X.; Xu, X.; Wang, Z.; Fang, P.; Mei, T. Chin. J. Org. Chem. 2020, 40, 3738. (in Chinese) |
| [1] | ( 王向阳, 徐学涛, 王振华, 方萍, 梅天胜, 有机化学, 2020, 40, 3738.) |
| [1] | (c) Yang, Q.; Yan, X.-T.; Feng, C.-T.; Chen, D.-X.; Yan, Z.-Z.; Xu, K. Org. Chem. Front. 2021, 8, 6384. |
| [1] | (d) Ge, Y.-X.; Yan, Q.-Q.; Tian, Y.-F.; Wang, H.-J.; Zhang, C.-F.; Li, Z.-J. Chin. J. Org. Chem. 2021, 41, 3106. (in Chinese) |
| [1] | ( 葛雅欣, 闫芹芹, 田云飞, 王海军, 张春芳, 李泽江, 有机化学, 2021, 41, 3106.) |
| [1] | (e) Yi, R.-N.; He, W.-M. Chin. J. Org. Chem. 2021, 41, 1267. (in Chinese) |
| [1] | ( 易荣楠, 何卫民, 有机化学, 2021, 41, 1267.) |
| [2] | Baran, P. S.; Corey, E. J. J. Am. Chem. Soc. 2002, 124, 7904. |
| [3] | (a) Wen, J.; Wang, F.; Zhang, X. M. Chem. Soc. Rev. 2021, 50, 3211. |
| [3] | (b) Jiang, X.-L.; Zhu, S.-L. Chin. J. Org. Chem. 2021, 41, 3745. (in Chinese) |
| [3] | ( 江晓莉, 朱少林, 有机化学, 2021, 41, 3745.) |
| [4] | (a) Zhang, M.; Zhang, Y.; Jie, X.; Zhao, H.; Li, G.; Su, W. P. Org. Chem. Front. 2014, 1, 843. |
| [4] | (b) Luo, H.; Pei, N.; Zhang, J. Chin. J. Org. Chem. 2021, 41, 2990. (in Chinese) |
| [4] | ( 罗欢欢, 裴娜, 张敬, 有机化学, 2021, 41, 2990.) |
| [4] | (c) Feng, Y.-L.; Shi, B. Chin. J. Org. Chem. 2021, 41, 3753. (in Chinese) |
| [4] | ( 冯亚岚, 史炳锋, 有机化学, 2021, 41, 3753.) |
| [4] | (d) Sun, S.-Z.; Wang, X.; Cheng, T.-J.; Xu, H.; Dai, H.-X. Chin. J. Org. Chem. 2020, 40, 3371 (in Chinese) |
| [4] | ( 孙尚政, 王星, 程泰锦, 徐辉, 戴辉雄, 有机化学, 2020, 40, 3371.) |
| [4] | (e) Liu, Y.-Y.; Zhang, Y.; Wan, J.-P. J. Org. Chem. 2017, 82, 8950. |
| [4] | (f) Du, Y.; Liu, Y.-Y.; Wan, J.-P. J. Org. Chem. 2018, 83, 3403. |
| [5] | (a) Wuts, P. G. M.; Greene, T. W. Protective Groups in Organic Synthesis, 4th ed., Wiley-Interscience, Hoboken, 2006, pp. 773-789. |
| [5] | (b) Yoo, M.; Jung, K.-W. ChemistrySelect 2018, 3, 1527. |
| [6] | Spaggiari, A.; Blaszczak, L. C.; Prati, F. Org. Lett. 2004, 6, 3885. |
| [7] | Koenig, S. G.; Vandenbossche, C. P.; Zhao, H.; Mousaw, P.; Singh, S. P.; Bakale, R. P. Org. Lett. 2009, 11, 433. |
| [8] | Sultane, P. R.; Mete, T. B.; Bhat, R. G. Org. Biomol. Chem. 2014, 12, 261. |
| [9] | Wang, A.-E.; Chang, Z.; Liu, Y.-P.; Huang, P.-Q. Chin. Chem. Lett. 2015, 26, 1055. |
| [10] | Kita, Y.; Nishii, Y.; Onoue, A.; Mashima, K. Adv. Synth. Catal. 2013, 355, 3391. |
| [11] | Shimizu, Y.; Morimoto, H.; Zhang, M.; Ohshima, T. Angew. Chem., Int. Ed. 2012, 51, 8564. |
| [12] | (a) Lian, F.; Xu, K. Chin. J. Org. Chem. 2020, 40, 3490. (in Chinese) |
| [12] | ( 廉菲, 徐坤, 有机化学, 2020, 40, 3490.) |
| [12] | (b) Meng, Z.-Y.; Feng, C.-T.; Xu, K. Chin. J. Org. Chem. 2021, 41, 2535. (in Chinese) |
| [12] | ( 蒙泽银, 冯承涛, 徐坤, 有机化学, 2021, 41, 2535.) |
| [12] | (c) Lian, F.; Xu, K.; Zeng, C. Chem. Rec. 2021, 21, 2290. |
| [12] | (d) Li, J.; Zhang, S.; Xu, K. Chin. Chem. Lett. 2021, 32, 2729. |
| [12] | (e) Zhang, S.; Li, L.; Li, J.; Shi, J.; Xu, K.; Gao, W.; Zong, L.; Li, G.; Findlater, M. Angew. Chem., Int. Ed. 2021, 60, 7275. |
| [12] | (f) Jiang, Y.; Xu, K.; Zeng, C. CCS Chem. 2021, 3, 1911. |
| [13] | Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Curr. Neuropharmacol. 2017, 15, 434. |
| [14] | Wang, Y.-H.; Tian, J.-S.; Tan, P.-W.; Cao, Q.; Zhang, X.-X.; Cao, Z.-Y.; Zhou, F.; Wang, X.; Zhou, J. Angew Chem., Int. Ed. 2020, 59, 1634. |
| [15] | Kerr, W. J.; Lindsay, D. M.; Owens, P. K.; Reid, M.; Tuttle, T.; Campos, S. ACS Catal. 2017, 7, 7182. |
| [16] | Roth, G. J.; Heckel, A.; Colbatzky, F.; Handschuh, S.; Kley, J.; Lintz, T. L.; Lotz, R.; Grunt, U. T.; Walter, R.; Hilberg, F. J. Med. Chem. 2009, 52, 4466. |
| [17] | Castelo-Branco, F. S.; Lima, E. C.; Domingos, J. L. O.; Pintob, A. C.; Lourenço, M. C. S.; Gomes, K. M.; Costa-Lima, M. M.; Araujo-Lima, C. F.; Aiub, C. A. F.; Felzenszwalb, I.; Costa, T. E. M. M.; Penido, C.; Henriques, M. G.; Boechat, N. Eur. J. Med. Chem. 2018, 146, 529. |
| [18] | He, K.-H.; Tan, F.-F.; Zhou, C.-Z.; Zhou, G.-J.; Yang, X.-L.; Li, Y. Angew. Chem., Int. Ed. 2017, 56, 3080. |
| [19] | Wang, Q.-F.; Chai, H.; Yu, Z.-K. Organometallics 2018, 37, 584. |
| [20] | Wu, J.-J.; Talwar, D.; Johnston, S.; Yan, M.; Xiao, J.-L. Angew. Chem., Int. Ed. 2013, 52, 6983. |
| [21] | Huang, Y.-Q.; Song, H.-J.; Liu, Y.-X.; Wang, Q.-M. Chem.-Eur. J. 2018, 24, 2065. |
| [22] | Zhang, J.-Y.; Chen, S.-Y.; Chen, F.-F.; Xu, W.-S.; Deng, G.-J.; Gong, H. Adv. Synth. Catal. 2017, 359, 2358. |
| [23] | Ning, X.-S.; Liang, X.; Hu, K.-F.; Yao, C.-Z.; Qu, J.-P.; Kang, Y.-B. Adv. Synth. Catal. 2018, 360, 1590. |
| [24] | Barykina, O. V.; Snider, B. B. Org. Lett. 2010, 12, 2664. |
| [25] | Song, W. Z.; Dong, K.; Li, M. Org. Lett. 2020, 22, 371. |
| [26] | Wang, M.; Fan, Q.-L.; Jiang, X.-F. Org. Lett. 2018, 20, 216. |
| [27] | Omer, H. M.; Liu, P.; Brummond, K. M. J. Org. Chem. 2020, 85, 7959. |
| [28] | Lin, W.; Hu, M.-H.; Feng, X.; Fu, L.; Cao, C.-P.; Huang, Z.-B.; Shi, D.-Q. Tetrahedron Lett. 2014, 55, 2238. |
| [29] | Laursen, S. R.; Jensen, M. T.; Lindhardt, A. T.; Jacobsen, M. F.; Skrydstrup, T. Eur. J. Org. Chem. 2016, 2016, 1881. |
| [30] | Zhang, F.; Li, L.; Zhang, J. Sci. Rep. 2019, 9, 2787. |
/
| 〈 |
|
〉 |