

钴催化的1-萘胺衍生物与α-羰基羧酸的脱羰C(8)-位酰氧基化反应
收稿日期: 2021-11-25
修回日期: 2022-01-14
网络出版日期: 2022-02-18
基金资助
浙江省自然科学基金(LQ15B020002); 绍兴市科技计划(2018C10017); 国家级大学生创新创业训练计划(201910349019)
Cobalt-Catalyzed Decarbonylative C(8)-Acyloxylation of 1-Naphthalamine Derivatives with α-Oxocarboxylic Acids
Received date: 2021-11-25
Revised date: 2022-01-14
Online published: 2022-02-18
Supported by
Zhejiang Provincial Natural Science Foundation(LQ15B020002); Shaoxing Science and Technology Plan Project(2018C10017); National College Students' Innovation and Entrepreneurship Training Program(201910349019)
王朝彧 , 董书达 , 朱天阳 , 刘玉琴 , 武梓涵 , 冯若昆 . 钴催化的1-萘胺衍生物与α-羰基羧酸的脱羰C(8)-位酰氧基化反应[J]. 有机化学, 2022 , 42(6) : 1799 -1810 . DOI: 10.6023/cjoc202111038
The cobalt-catalyzed decarbonylative C(8)-acyloxylation of 1-naphthalamine derivatives with α-oxocarboxylic acids in the presence of Ag2CO3 and Na2CO3 was developed. Various substituted phenylglyoxylic acids, naphthalenylglyoxylic acid and 2-oxo-2-(thiophen-2-yl)acetic acid can be tolerated in this reaction, giving the desired products in moderate yields. In addition, when deuterated allyl glyoxylic acid is used, the yield of isotope labeled aromatic esterification product is 62%.
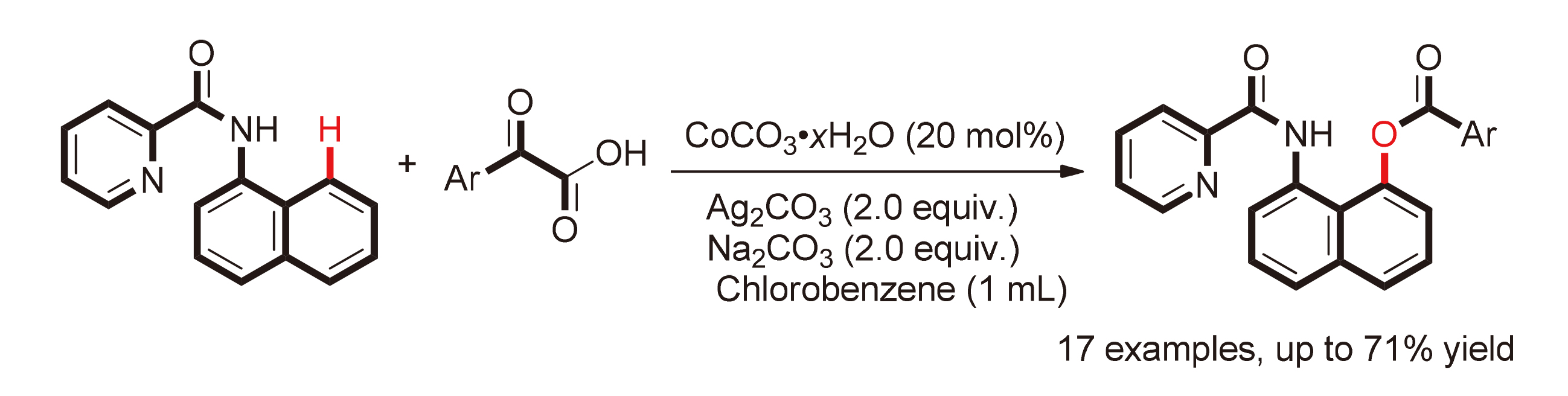
| [1] | (a) Durward, W.; Cruickshank, J.; Sparks, R. A. Proc. R. Soc. London. Ser. A 1960, 258, 270. |
| [1] | (b) Prévost, S. ChemPlusChem 2020, 85, 476. |
| [2] | O'Reilly, M.; Kirkwood, N. K.; Kenyon, E. J.; Huckvale, R.; Cantillon, D. M.; Waddell, S. J.; Ward, S. E.; Richardson, G. P.; Kros, C. J.; Derudas, M. J. Med. Chem. 2019, 62, 5312. |
| [3] | Panchgalle, S. P.; Gore, R. G.; Chavan, S. P.; Kalkote, U. R. Tetrahedron 2009, 20, 1767. |
| [4] | Example for Recent Advances in Directing Group-Induced C-H Activation Reactions, see: (a) Ujwaldev, S. M.; Harry, N. A.; Divakar, M. A.; Anilkumar, G. Catal. Sci. Technol. 2018, 8, 5983. |
| [4] | (b) Wang, S.; Yan, F.; Wang, L.; Zhu, L. Chin. J. Org. Chem. 2018, 38, 291. (in Chinese) |
| [4] | 汪珊, 严沣, 汪连生, 朱磊, 有机化学, 2018, 38, 291.). |
| [4] | (c) Guan, Z.-H.; Usman, M.; Ren, Z.-H.; Wang, Y.-Y. Synthesis 2017, 49, 1419. |
| [5] | For selected papers on C-2 functionalizations of 1-naphthylamide derivatives, see: (a) Daugulis, O.; Zaitsev, V. G. Angew. Chem., Int. Ed. 2005, 44, 4046. |
| [5] | (b) Kim, B. S.; Jang, C.; Lee, D. J.; Youn, S. W. Chem. Asian J. 2010, 5, 2336. |
| [5] | (c) Yip, K.-T.; Yang, D. Org. Lett. 2011, 13, 2134. |
| [5] | (d) Wu, Y.; Choy, P. Y.; Mao, F.; Kwong, F. Y. Chem. Commun. 2013, 49, 689. |
| [5] | (e) Szabó, F.; Daru, J.; Simkó, D.; Nagy, T. Z.; Stirling, A.; Novák, Z. Adv. Synth. Catal. 2013, 355, 685. |
| [5] | (f) Gao, Y.; Huang, Y.; Wu, W.; Huang, K.; Jiang, H. Chem. Commun. 2014, 50, 8370. |
| [5] | (g) Li, Q.; Zhang, S.-Y.; He, G.; Ai, Z.; Nack, W. A.; Chen, G. Org. Lett. 2014, 16, 1764. |
| [5] | (h) Iwasaki, M.; Iyanaga, M.; Tsuchiya, Y.; Nishimura, Y.; Li, W.; Li, Z.; Nishihara, Y. Chem.-Eur. J. 2014, 20, 2459. |
| [5] | (h) Zhang, X.; Si, W.; Bao, M.; Asao, N.; Yamamoto, Y.; Jin, T. Org. Lett. 2014, 16, 4830. |
| [5] | (i) Das, R.; Kapur, M. J. Org. Chem. 2017, 82, 1114. |
| [5] | (j) Li, Z.-L.; Sun, K.-K.; Cai, C. Org. Biomol. Chem. 2018, 16, 5433. |
| [5] | (k) Wu, P.; Huang, W.; Cheng, T.-J.; Lin, H.-X.; Dai, H.-X. Org. Lett. 2020, 22, 5051. |
| [5] | (l) Li, Q.; Huang, J.; Chen, G.; Wang, S.-B. Org. Biomol. Chem. 2020, 18, 4802. |
| [5] | (m) Sarkar, S.; Sahoo, T.; Sen, C.; Ghosh, S. C. Chem. Commun. 2021, 57, 8949. |
| [5] | (n) Wang, D.; Xu, X.; Zhang, J.; Zhao, Y. J. Org. Chem. 2021, 86, 2696. |
| [6] | For selected papers on C-4-functionalizations of 1-naphthylamide derivatives, see: (a) Li, J.-M.; Wang, Y.-H.; Yu, Y.; Wu, R.-B.; Weng, J.; Lu, G. ACS Catal. 2017, 7, 2661. |
| [6] | (b) Liang, S.; Bolte, M.; Manolikakes, G. Chem.-Eur. J. 2017, 23, 96. |
| [6] | (c) Bai, P.; Sun, S.; Li, Z.; Qiao, H.; Su, X.; Yang, F.; Wu, Y. Wu, Y. J. Org. Chem. 2017, 82, 12119. |
| [6] | (d) Han, S.; Liang, A.; Ren, X.; Gao, X.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2017, 58, 4859. |
| [6] | (e) You, G.; Wang, K.; Wang, X.; Wang, G.; Sun, J.; Duan, G.; Xia, C. Org. Lett. 2018, 20, 4005. |
| [6] | (f) Zhu, H.; Sun, S.; Qiao, H.; Yang, F.; Kang, J.; Wu, Y.; Wu, Y. Org. Lett. 2018, 20, 620. |
| [6] | (g) Dou, Y.; Yin, B.; Zhang, P.; Zhu, Q. Eur. J. Org. Chem. 2018, 4571. |
| [6] | (h) Xu, J.; Du, K.; Shen, J.; Shen, C.; Chai, K.; Zhang, P. ChemCatChem 2018, 10, 3675. |
| [6] | (i) Zhao, L.; Sun, M.; Yang, F,; Wu, Y. J. Org. Chem. 2021, 86, 11519. |
| [7] | Example for Precious metal catalyzed C-8-functionalizations of 1-naphthylamide derivativesl, see: (a) Huang, L.; Li, Q.; Wang, C.; Qi, C. J. Org. Chem. 2013, 78, 3030. |
| [7] | (b) Huang, L.; Sun, X.; Li, Q.; Qi, C. J. Org. Chem. 2014, 79, 6720. |
| [7] | (c) Iwasaki, M.; Kaneshika, W.; Tsuchiya, Y. J. Org. Chem. 2014, 79, 11330. |
| [7] | (d) Li, Z.; Sun, S.; Qiao, H.; Yang, F.; Zhu, Y.; Kang, J.; Wu, Y.; Wu, Y. Org. Lett. 2016, 18, 4594. |
| [7] | (e) Zhu, H.; Sun, S.; Qiao, H.; Yang, F.; Kang, J.; Wu, Y.; Wu, Y. Org. Lett. 2018, 20, 620. |
| [7] | (f) Han, J.-N.; Du, C.; Zhu, X.; Wang, Z.-L.; Zhu, Y.; Chu, Z.-Y.; Niu, J.-L.; Song, M.-P. Beilstein J. Org. Chem. 2018, 14, 2090. |
| [7] | (g) Yu, X.; Yang, F.; Wu, Y.; Wu, Y. Org. Lett. 2019, 21, 1726. |
| [7] | (h) Zhang, T.; Zhu, H.; Yang, F.; Wu, Y.; Wu, Y. Tetrahedron 2019, 75, 1541. |
| [7] | (i) Zhang, M.; Li, R.; Yang, Z.; Feng, R. Chin. J. Org. Chem. 2020, 40, 714. (in Chinese) |
| [7] | ( 张梦帆, 李瑞鹏, 杨震, 冯若昆, 有机化学, 2020, 40, 714.) |
| [7] | (j) Gao, Y.; Zhang, M.; Wang, C.; Yang, Z.; Huang, X.; Feng, R.; Qi, C. Chem. Commun. 2020, 56, 14231. |
| [7] | (k) Zu, C.; Zhang, T.; Yang, F.; Wu, Y.; Wu, Y. J. Org. Chem. 2020, 85, 12777. |
| [7] | (l) Shi, Y.; Yang, F.; Wu, Y. Org. Biomol. Chem. 2020, 18, 4628. |
| [7] | (m) Zu, C.; Zhang, T.; Yang, F.; Wu, Y.; Wu, Y. J. Org. Chem. 2020, 85, 12777. |
| [7] | (n) Sun, Y.; Feng, C.; Wang, P.; Yang, F.; Wu, Y. Org. Chem. Front. 2021, 8, 5710. |
| [8] | Recent reviews for Cross-Dehydrogenative Couplings CDC, see: (a) Zhang, Y.; Feng, B. Chin. J. Org. Chem. 2014, 34, 2406. (in Chinese) |
| [8] | ( 张艳, 冯柏年, 有机化学, 2014, 34, 2406.) |
| [8] | (b) Krylov, I. B.; Vil’, V. A.; Terent'ev, A. O. Beilstein J. Org. Chem. 2015, 11, 92. |
| [8] | (c) Arshadi, S.; Banaei, A.; Monfared, A.; Ebrahimiaslbc, S.; Hosseinian, A. RSC Adv. 2019, 9, 17101. |
| [8] | (d) Peng, W.; Vessally, E.; Arshadi, S.; Monfared, A.; Hosseinian, A.; Edjlali, L. Top. Curr. Chem. 2019, 377, 20. |
| [8] | (e) Peng, K.; Dong, Z. Adv. Synth. Catal. 2021, 363, 1185. |
| [8] | (f) Afsina, C. M. A.; Aneeja, T.; Neetha, M.; Anikumar, G. Eur. J. Org. Chem. 2021, 1776. |
| [9] | Raghuvanshi, K.; Zell, D.; Ackermann, L. Org. Lett. 2017, 19, 1278. |
| [10] | Ye, Z.; Wang, W.; Luo, F.; Zhang, S.; Cheng, J. Org. Lett. 2009, 11, 3974. |
| [11] | Wu, Y.; Zhou, B. Org. Lett. 2017, 1, 3532. |
| [12] | Lin, C.; Chen, Z.; Liu, Z.; Zhang, Y. Adv. Synth. Catal. 2018, 360, 519. |
| [13] | Ueno, R.; Natsui, S.; Chatani, N. Org. Lett. 2018, 20, 1062. |
| [14] | Lan, J.; Xie, H.; Lu, X.; Deng, Y.; Jiang, H.; Zeng, W. Org. Lett. 2017, 19, 4279. |
| [15] | Example for preparation of aryl ketones through decarboxylative cross-coupling, see: (a) Guo, L.-N.; Wang, H.; Duan, X.-H. Org. Biol. Chem. 2016, 14, 7380. |
| [15] | (b) Huang, F.; Chen, X.; Xie, Y.; Zeng, W. Chin. J. Org. Chem. 2017, 37, 3. (in Chinese) |
| [15] | ( 黄房生, 陈训, 谢应, 曾伟, 有机化学, 2017, 37, 31.) |
| [15] | (c) Ruan, L.; Chen, C.; Zhang, X.; Jing, S. Chin. J. Org. Chem. 2018, 38, 3155. (in Chinese) |
| [15] | ( 阮利衡, 陈春欣, 张晓欣, 孙京, 有机化学, 2018, 38, 3155.) |
| [15] | (d) Bao, P.; Liu, F.; Lv, Y.; Yue, H.; Li, J.; Wei, W. Org. Chem. Front. 2020, 7, 492. |
| [15] | (e) Lalji, R.; Kumar, P.; Gupta, M.; Parmar, V.; Singh, B. Adv. Synth. Catal. 2020, 362, 552. |
| [15] | (f) Ni, H.; Shi, X.; Li, Y.; Zhang, X.; Zhao, J.; Zhao, F. Org. Biol. Chem. 2020, 18, 6558. |
| [15] | (g) Waghmare, D.-S.; Tambe, S.-D.; Kshirsagar, U.-A. Asian J. Org. Chem. 2020, 9, 2095. |
| [15] | (h) Hou, C.; Sun, S.; Liu, Z.; Zhang, H.; Liu, Y.; An, Q.; Zhao, J.; Ma, J.; Sun, Z.; Chu, W. Adv. Synth. Catal. 2021, 363, 2806. |
| [16] | Chen, R.; Zeng, L.; Huang, B.; Shen, Y.; Cui, S. Org. Lett. 2018, 20, 3377. |
| [17] | CCDC 2141840 contains the supplementary crystallographic data for this paper. These data are available free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/ data_request/cif. |
| [18] | Zhao, D.; Luo, H.; Chen, B.; Chen, W.; Zhang, G.; Yu, Y. J. Org. Chem. 2018, 83, 7860. |
| [19] | Lozano, D.; Álvarez-Yebra, R.; López-Coll, R.; Lledó, A. Chem. Sci. 2019, 10, 10351. |
| [20] | Example for the mechanism of SET, see: (a) Zhang, L. B.; Hao, X. Q.; Zhang, S. K.; Liu, Z. J.; Zheng, X. X.; Gong, J. F.; Niu, J. L.; Song, M. P. Angew. Chem., Int. Ed. 2015, 54, 272. |
| [20] | (b) Tan, G.; He, S.; Huang, X.; Liao, X.; Cheng, Y.; You, J. Angew. Chem., Int. Ed. 2016, 55, 10414. |
| [20] | (c) Guo, X. K.; Zhang, L. B.; Wei, D.; Niu, J. L. Chem. Sci. 2015, 6, 7059. |
| [21] | Xie, Y.; Yang, Y.; Huang, L.; Zhang, X.; Zhang, Y. Org. Lett. 2012, 14, 1238. |
| [22] | Wadhwa, K.; Yang, C.; West, P. R.; Deming, K. C.; Chemburkar, S. R.; Reddy, R. E. Synth. Commun. 2008, 38, 4434. |
/
| 〈 |
|
〉 |