

通过电化学Appel反应合成腈
收稿日期: 2022-02-06
修回日期: 2022-03-11
网络出版日期: 2022-03-22
基金资助
国家自然科学基金(22001217); 四川省科技厅(2021ZYD0064); 西华师范大学英才基金(17YC020); 西华师范大学基本科研业务费(19D037); 西华师范大学基本科研业务费(19E034)
Synthesis of Nitrile via Electrochemical Appel Reaction
Received date: 2022-02-06
Revised date: 2022-03-11
Online published: 2022-03-22
Supported by
National Natural Science Foundation of China(22001217); Science and Technology Program of Sichuan Province(2021ZYD0064); Meritocracy Research Funds of China West Normal University(17YC020); Fundamental Research Funds of China West Normal University(19D037); Fundamental Research Funds of China West Normal University(19E034)
李海琼 , 尹梦云 , 谢芬芬 , 张正兵 , 韩盼 , 敬林海 . 通过电化学Appel反应合成腈[J]. 有机化学, 2022 , 42(7) : 2229 -2235 . DOI: 10.6023/cjoc202202007
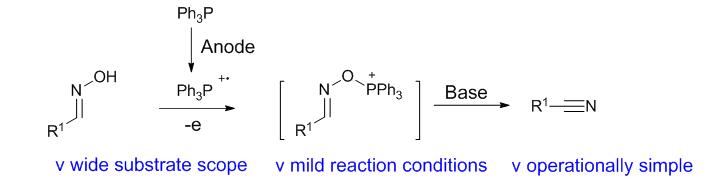
An electrochemical Appel reaction was developed for the synthesis of nitriles. This protocol features operationally simplicity, mild reaction conditions and environmental friendliness, enabling synthesis of various aromatic and aliphatic nitriles. Based on the controlled experiments and cyclic voltammetry (CV) experimental results, an electrochemical Appel reaction mechanism was proposed to explain the reaction process.
| [1] | (a) Dworczak, R.; Fabian, W. M. F.; Biza, P.; Weikmann, M.; Junek, H. Dyes Pigm. 1995, 28, 297. |
| [1] | (b) Dworczak, R.; Fabian, W. M. F.; Pawar, B. N.; Junek, H. Dyes Pigm. 1995, 29, 65. |
| [1] | (c) Pearce, E. M.; Weil, E. D.; Barinov, V. Y. Fire Smart Polymers (Fire and Polymers), American Chemical Society, 2001, pp. 37-48. |
| [1] | (d) Amr, M. A.; Mohamed, H. E.; Mohamed, S. E.; Hesham, R. E.-S.; Ismail, A. A. Curr. Org. Synth. 2018, 15, 487. |
| [2] | Fatiadi, A. J. In Preparation and Synthetic Applications of Cyano Compounds, Eds.: Patai, S.; Rappaport, Z., Wiley, New York, 1983. |
| [3] | (a) Iyengar, B. S.; Dorr, R. T.; Remers, W. A. J. Med. Chem. 2004, 47, 218. |
| [3] | (b) Romero, M.; Renard, P.; Caignard, D.-H.; Atassi, G.; Solans, X.; Constans, P.; Bailly, C.; Pujol, M. D. J. Med. Chem. 2007, 50, 294. |
| [4] | Pascual, E.; Sivera, F.; Yasothan, U.; Kirkpatrick, P. Nat. Rev. Drug Discov. 2009, 8, 191. |
| [5] | Patat, A.; Paty, I.; Hindmarch, I. Hum. Psychopharmacol. 2001, 16, 369. |
| [6] | (a) Sica, D. A.; Prisant, L. M. J. Clin. Hypertens. (Shelton, CT, U. S.) 2007, 9, 1. |
| [6] | (b) Cooper-DeHoff, R. M.; Handberg, E. M.; Mancia, G.; Zhou, Q.; Champion, A.; Legler, U. F.; Pepine, C. J. Expert Rev. Cardiovasc. Ther. 2009, 7, 1329. |
| [7] | Noble, S.; McTavish, D. Drugs 1995, 50, 1032. |
| [8] | (a) Shu, Z.; Ye, Y.; Deng, Y.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2013, 52, 10573. |
| [8] | (b) Liu, J.; Zheng, H.-X.; Yao, C.-Z.; Sun, B.-F.; Kang, Y.-B. J. Am. Chem. Soc. 2016, 138, 3294. |
| [8] | (c) Ge, J.-J.; Yao, C.-Z.; Wang, M.-M.; Zheng, H.-X.; Kang, Y.-B.; Li, Y. Org. Lett. 2016, 18, 228. |
| [8] | (d) Yu, L.; Li, H.; Zhang, X.; Ye, J.; Liu, J.; Xu, Q.; Lautens, M. Org. Lett. 2014, 16, 1346. |
| [8] | (e) Zhuang, Y.-J.; Liu, J.; Kang, Y.-B. Tetrahedron Lett. 2016, 57, 5700. |
| [9] | (a) Zhou, S.; Addis, D.; Das, S.; Junge, K.; Beller, M. Chem. Commun. 2009, 4883. |
| [9] | (b) Shipilovskikh, S. A.; Vaganov, V. Y.; Denisova, E. I.; Rubtsov, A. E.; Malkov, A. V. Org. Lett. 2018, 20, 728. |
| [10] | (a) Zhou, W.; Zhang, L.; Jiao, N. Angew. Chem., Int. Ed. 2009, 48, 7094. |
| [10] | (b) Tseng, K.-N. T.; Rizzi, A. M.; Szymczak, N. K. J. Am. Chem. Soc. 2013, 135, 16352. |
| [10] | (c) Guo, S.; Wan, G.; Sun, S.; Jiang, Y.; Yu, J.-T.; Cheng, J. Chem. Commun. 2015, 51, 5085. |
| [11] | (a) Anderson, B. A.; Bell, E. C.; Ginah, F. O.; Harn, N. K.; Pagh, L. M.; Wepsiec, J. P. J. Org. Chem. 1998, 63, 8224. |
| [11] | (b) Zanon, J.; Klapars, A.; Buchwald, S. L. J. Am. Chem. Soc. 2003, 125, 2890. |
| [11] | (c) Cristau, H.-J.; Ouali, A.; Spindler, J.-F.; Taillefer, M. Chem.-Eur. J. 2005, 11, 2483. |
| [11] | (d) Pan, S.; Wu, F.; Yu, R.; Chen, W. J. Org. Chem. 2016, 81, 1558. |
| [11] | (e) Yan, G.; Zhang, Y.; Wang, J. Adv. Synth. Catal. 2017, 359, 4068. |
| [12] | (a) Fang, C.; Li, M.; Hu, X.; Mo, W.; Hu, B.; Sun, N.; Jin, L.; Shen, Z. RSC Adv. 2017, 7, 1484. |
| [12] | (b) Murugesan, K.; Senthamarai, T.; Sohail, M.; Sharif, M.; Kalevaru, N. V.; Jagadeesh, R. V. Green Chem. 2018, 20, 266. |
| [12] | (c) Chen, H.; Sun, S.; Xi, H.; Hu, K.; Zhang, N.; Qu, J.; Zhou, Y. Tetrahedron Lett. 2019, 60, 1434. |
| [12] | (d) Zhan, W.; Tong, M.; Ji, L.; Zhang, H.; Ge, Z.; Wang, X.; Li, R.. Chin. Chem. Lett. 2019, 30, 973. |
| [12] | (e) Mudshinge, S. R.; Potnis, C. S.; Xu, B.; Hammond, G. B. Green Chem. 2020, 22, 4161. |
| [12] | (f) Hua, M.; Song, J.; Huang, X.; Liu, H.; Fan, H.; Wang, W.; He, Z.; Liu, Z.; Han, B. Angew. Chem., Int. Ed. 2021, 60, 21479. |
| [13] | (a) Xu, J.-H.; Jiang, Q.; Guo, C.-C. J. Org. Chem. 2013, 78, 11881. |
| [13] | (b) Preger, Y.; Root, T. W.; Stahl, S. S. ACS Omega 2018, 3, 6091. |
| [13] | (c) Vanoye, L.; Hammoud, A.; Gérard, H.; Barnes, A.; Philippe, R.; Fongarland, P.; de Bellefon, C.; Favre-Réguillon, A. ACS Catal. 2019, 9, 9705. |
| [13] | (d) Murata, Y.; Iwasa, H.; Matsumura, M.; Yasuike, S. Chem. Pharm. Bull. 2020, 68, 679. |
| [13] | (e) Takahashi, Y.; Tsuji, H.; Kawatsura, M. J. Org. Chem. 2020, 85, 2654. |
| [13] | (f) Lu, D.; Cui, J.; Yang, S.; Gong, Y. ACS Catal. 2021, 11, 4288. |
| [13] | (g) Xiao, J.; Guo, F.; Li, Y.; Li, F.; Li, Q.; Tang, Z.-L. J. Org. Chem. 2021, 86, 2028. |
| [14] | (a) Li, Y.-T.; Liao, B.-S.; Chen, H.-P.; Liu, S.-T. Synthesis 2011, 2639. |
| [14] | (b) Ma, X.-Y.; He, Y.; Lu, T.-T.; Lu, M. Tetrahedron 2013, 69, 2560. |
| [14] | (c) Ghosh, P.; Pariyar, G. C.; Saha, B.; Subba, R. Synth. Commun. 2016, 46, 685. |
| [14] | (d) Hyodo, K.; Kitagawa, S.; Yamazaki, M.; Uchida, K. Chem. Asian J. 2016, 11, 1348. |
| [14] | (e) Rapeyko, A.; Climent, M. J.; Corma, A.; Concepción, P.; Iborra, S. ACS Catal. 2016, 6, 4564. |
| [14] | (f) Sun, D.; Kitamura, E.; Yamada, Y.; Sato, S. Green Chem. 2016, 18, 3389. |
| [14] | (g) Ding, R.; Liu, Y.; Han, M.; Jiao, W.; Li, J.; Tian, H.; Sun, B. J. Org. Chem. 2018, 83, 12939. |
| [14] | (h) Zhang, D.; Huang, Y.; Zhang, E.; Yi, R.; Chen, C.; Yu, L.; Xu, Q. Adv. Synth. Catal. 2018, 360, 784. |
| [14] | (i) Ma, X.; Liu, D.; Chen, Z. Synth. Commun. 2021, 51, 3261. |
| [15] | (a) Sperry, J. B.; Wright, D. L. Chem. Soc. Rev. 2006, 35, 605. |
| [15] | (b) Jutand, A. Chem. Rev. 2008, 108, 2300. |
| [15] | (c) Yoshida, J.-I.; Kataoka, K.; Horcajada, R.; Nagaki, A. Chem. Rev. 2008, 108, 2265. |
| [15] | (d) Francke, R.; Little, R. D. Chem. Soc. Rev. 2014, 43, 2492. |
| [15] | (e) Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230. |
| [15] | (f) Ma, C.; Fang, P.; Mei, T.-S. ACS Catal. 2018, 8, 7179. |
| [15] | (g) Moeller, K. D. Chem. Rev. 2018, 118, 4817. |
| [15] | (h) Yoshida, J.-I.; Shimizu, A.; Hayashi, R. Chem. Rev. 2018, 118, 4702. |
| [15] | (i) Marken, F.; Wadhawan, J. D. Acc. Chem. Res. 2019, 52, 3325. |
| [15] | (j) Xiong, P.; Xu, H.-C. Acc. Chem. Res. 2019, 52, 3339. |
| [15] | (k) Yuan, Y.; Lei, A. Acc. Chem. Res. 2019, 52, 3309. |
| [15] | (l) Jiao, K.-J.; Xing, Y.-K.; Yang, Q.-L.; Qiu, H.; Mei, T.-S. Acc. Chem. Res. 2020, 53, 300. |
| [15] | (m) Siu, J. C.; Fu, N.; Lin, S. Acc. Chem. Res. 2020, 53, 547. |
| [16] | (a) Mo, Z.-Y.; Swaroop, T. R.; Tong, W.; Zhang, Y.-Z.; Tang, H.-T.; Pan, Y.-M.; Sun, H.-B.; Chen, Z.-F. Green Chem. 2018, 20, 4428. |
| [16] | (b) Zhang, Y.-Z.; Mo, Z.-Y.; Wang, H.-S.; Wen, X.-A.; Tang, H.-T.; Pan, Y.-M. Green Chem. 2019, 21, 3807. |
| [16] | (c) Wang, X.-Y.; Zhong, Y.-F.; Mo, Z.-Y.; Wu, S.-H.; Xu, Y.-L.; Tang, H.-T.; Pan, Y.-M. Adv. Synth. Catal. 2021, 363, 208. |
| [16] | (d) Wu, Y.; Chen, J.-Y.; Liao, H.-R.; Shu, X.-R.; Duan, L.-L.; Yang, X.-F.; He, W.-M. Green Synth. Catal. 2021, 2, 233. |
| [16] | (e) Yang, Z.; Yu, Y.; Lai, L.; Zhou, L.; Ye, K.; Chen, F.-E. Green Synth. Catal. 2021, 2, 19. |
| [16] | (f) Zhang, S.; Ye, X.; Wojtas, L.; Hao, W.; Shi, X. Green Synth. Catal. 2021, 2, 82. |
| [17] | Libendi, S. S.; Demizu, Y.; Onomura, O. Org. Biomol. Chem. 2009, 7, 351. |
| [18] | (a) Cui, T.; Zhan, Y.; Dai, C.; Lin, J.; Liu, P.; Sun, P. J. Org. Chem. 2021, 86, 15897. |
| [18] | (b) Gao, J.; Weng, X.; Ma, C.; Xu, X.; Fang, P.; Mei, T. Chin. J. Org. Chem. 2021, 41, 3223. (in Chinese) |
| [18] | ( 高君青, 翁信军, 马聪, 徐学涛, 方萍, 梅天胜, 有机化学, 2021, 41, 3223.) |
| [19] | Dai, J.-J.; Huang, Y.-B.; Fang, C.; Guo, Q.-X.; Fu, Y. ChemSusChem 2012, 5, 617. |
| [20] | Ye, J.-Q.; Zhang, Z.-L.; Zha, Z.-G.; Wang, Z.-Y. Chin. Chem. Lett. 2014, 25, 1112. |
| [21] | Qu, Q.; Gao, X.; Gao, J.; Yuan, G. Sci. China Chem. 2015, 58, 747. |
| [22] | Shono, T.; Matsumura, Y.; Tsubata, K.; Kamada, T.; Kishi, K.. J. Org. Chem. 1989, 54, 2249. |
| [23] | Hartmer, M. F.; Waldvogel, S. R. Chem. Commun. 2015, 51, 16346. |
| [24] | (a) Ohmori, H.; Nakai, S.; Sekiguchi, M.; Masui, M. Chem. Pharm. Bull. 1980, 28, 910. |
| [24] | (b) Xu, Z.; Zheng, Y.; Wang, Z.; Shao, X.; Tian, L.; Wang, Y. Chem. Commun. 2019, 55, 15089. |
| [25] | (a) de Andrade, V. S. C.; de Mattos, M. C. S. Curr. Org. Synth. 2015, 12, 309. |
| [25] | (b) Li, Z.; Sun, W.; Wang, X.; Li, L.; Zhang, Y.; Li, C. J. Am. Chem. Soc. 2021, 143, 3536. |
| [26] | (a) Ban, Y.-L.; Dai, J.-L.; Jin, X.-L.; Zhang, Q.-B.; Liu, Q. Chem. Commun. 2019, 55, 9701. |
| [26] | (b) Schäfer, R. J. B.; Monaco, M. R.; Li, M.; Tirla, A.; Rivera- Fuentes, P.; Wennemers, H. J. Am. Chem. Soc. 2019, 141, 18644. |
/
| 〈 |
|
〉 |