

1,3-二氢-3-亚烷基-吲哚-2-酮的膦催化合成研究
收稿日期: 2022-01-19
修回日期: 2022-03-16
网络出版日期: 2022-03-30
基金资助
国家自然科学基金(21772151); 国家自然科学基金(22072111)
Phosphine-Catalyzed Synthesis of 1,3-Dihydro-3-alkylidene-2H-indol-2-ones
Received date: 2022-01-19
Revised date: 2022-03-16
Online published: 2022-03-30
Supported by
National Natural Science Foundation of China(21772151); National Natural Science Foundation of China(22072111)
李婧婕 , 王宇豪 , 孟甜甜 , 黄毅勇 . 1,3-二氢-3-亚烷基-吲哚-2-酮的膦催化合成研究[J]. 有机化学, 2022 , 42(7) : 2222 -2228 . DOI: 10.6023/cjoc202201030
A P(4-OMe-C6H4)3-catalyzed Michael addition/double bond migration tandem reaction between allenyl imide and indol-2-ones was developed. This synthetic strategy for diverse 1,3-dihydro-3-alkylidene-2H-indol-2-ones features operationally simple, good substrate scope, high chemoselectivity and mild reaction conditions. The proposed reaction mechanism is also provided.
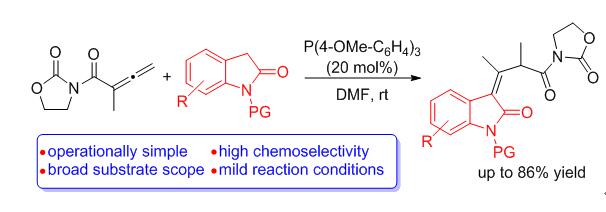
Key words: indol-2-one; allene; phosphine catalysis; Michael addition; double bond migration
| [1] | Singh, G. S.; Desta, Z. Y. Chem. Rev. 2012, 112, 6104. |
| [2] | (a) Sun, L.; Tran, N.; Tang, F.; App, H.; Hirth, P.; McMahon, G.; Tang, C. J. Med. Chem. 1998, 41, 2588. |
| [2] | (b) Andreani, A.; Burnelli, S.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Varoli, L.; Kunkel, M. W. J. Med. Chem. 2006, 49, 6922. |
| [2] | (c) Zhang, W.; Go, M.-L. Bioorg. Med. Chem. 2009, 17, 2077. |
| [2] | (d) Millemaggi, A.; Taylor, R. J. K. Eur. J. Org. Chem. 2010, 2010, 4527. |
| [3] | Ndolo, K. M.; An, S. J.; Park, K. R.; Lee, H. J.; Yoon, K. B.; Kim, Y.-C.; Han, S.-Y. Biomol. Ther. 2019, 27, 216. |
| [4] | Jang, J.-P.; Nogawa, T.; Uramoto, M.; Okano, A.; Futamura, Y.; Shimizu, T.; Takahashi, S.; Jang, J.-H.; Ahn, J. S.; Osada, H. J. Antibiot. 2015, 68, 293. |
| [5] | Otterness, I. G.; Bliven, M. L.; Downs, J. T.; Natoli, E. J.; Hanson, D. C. Cytokine 1991, 3, 277. |
| [6] | Roth, G. J.; Heckel, A.; Colbatzky, F.; Handschuh, S.; Kley, J.; Lehmann-Lintz, T.; Lotz, R.; Tontsch-Grunt, U.; Walter, R.; Hilberg, F. J. Med. Chem. 2009, 52, 4466. |
| [7] | Sun, L.; Liang, C.; Shirazian, S.; Zhou, Y.; Miller, T.; Cui, J.; Fukuda, J. Y.; Chu, J.-Y.; Nematalla, A.; Wang, X.; Chen, H.; Sistla, A.; Luu, T. C.; Tang, F.; Wei, J.; Tang, C. J. Med. Chem. 2003, 46, 1116. |
| [8] | (a) Ball-Jones, N. R.; Badillo, J. J.; Franz, A. K. Org. Biomol. Chem. 2012, 10, 5165. |
| [8] | (b) Boddy, A. J.; Bull, J. A. Org. Chem. Front. 2021, 8, 1026. |
| [8] | (c) Mei, G.-J.; Shi, F. Chem. Commun. 2018, 54, 6607. |
| [9] | (a) Dhondge, A. P.; Huang, Y.-X.; Lin, T.; Hsu, Y.-H.; Tseng, S.-L.; Chang, Y.-C.; Chen, H. J. H.; Kuo, M.-Y. J. Org. Chem. 2019, 84, 14061. |
| [9] | (b) Salem, M. A.; Ragab, A.; Askar, A. A.; El-Khalafawy, A.; Makhlouf, A. H. Eur. J. Med. Chem. 2020, 188, 111977. |
| [9] | (c) Li, G.; Feng, X.; Du, H. Org. Biomol. Chem. 2015, 13, 5826. |
| [9] | (d) Sharma, P.; Thummuri, D.; Reddy, T. S.; Senwar, K. R.; Naidu, V. G. M.; Srinivasulu, G.; Bharghava, S. K.; Shankaraiah, N. Eur. J. Med. Chem. 2016, 122, 584. |
| [9] | (e) Lathourakis, G. E.; Litinas, K. E. J. Chem. Soc., Perkin Trans. 1 1996, 491. |
| [9] | (f) Maigali, S. S.; El-Hussieny, M.; Soliman, F. M. J. Heterocycl. Chem. 2015, 52, 15. |
| [9] | (g) Rossetti, A.; Sacchetti, A.; Bonfanti, M.; Roda, G.; Rainoldi, G.; Silvani, A. Tetrahedron 2017, 73, 4584. |
| [10] | (a) Methot, J. L.; Roush, W. R. Adv. Synth. Catal. 2004, 346, 1035. |
| [10] | (b) Cai, W.; Huang, Y. Chin. J. Org. Chem. 2021, 41, 3903. (in Chinese) |
| [10] | ( 蔡卫, 黄有, 有机化学, 2021, 41, 3903.) |
| [10] | (c) Wei, Y.; Shi, M. Org. Chem. Front. 2017, 4, 1876. |
| [10] | (d) Ni, H.-Z.; Chan, W.-L.; Lu, Y.-X. Chem. Rev. 2018, 118, 9344. |
| [10] | (e) Guo, H.-C.; Fan, Y.-C.; Sun, Z.-H.; Wu, Y.; Kwon, O. Chem. Rev. 2018, 118, 10049. |
| [10] | (f) Li, E.-Q.; Huang, Y. Chem. Commun. 2020, 56, 680. |
| [11] | (a) Lu, X.; Zhang, C.; Xu, Z. Acc. Chem. Res. 2001, 34, 535. |
| [11] | (b) Lu, X. Chin. J. Org. Chem. 2001, 21, 769. (in Chinese) |
| [11] | ( 陆熙炎, 有机化学, 2001, 21, 769.) |
| [11] | (c) Tang, Q.; Tu, A.; Deng, Z.; Hu, M.; Zhong, W. Chin. J. Org. Chem. 2013, 33, 954. (in Chinese) |
| [11] | ( 唐谦, 涂爱平, 邓真真, 胡梦莹, 钟为慧, 有机化学, 2013, 33, 954.) |
| [11] | (d) Zhou, R.; Liu, R.; Li, R.; He, Z. Chin. J. Org. Chem. 2014, 34, 2385. (in Chinese) |
| [11] | ( 周荣, 刘蓉芳, 李瑞丰, 贺峥杰, 有机化学, 2014, 34, 2385.) |
| [11] | (e) Nallapati, S. B.; Chuang, S.-C. Asian J. Org. Chem. 2018, 7, 1743. |
| [12] | (a) Szeto, J.; Sriramurthy, V.; Kwon, O. Org. Lett. 2011, 13, 5420. |
| [12] | (b) Li, E.; Xie, P.; Yang, L.; Liang, L.; Huang, Y. Chem.-Asian J. 2013, 8, 603. |
| [12] | (c) Gandi, V. R.; Lu, Y. Chem. Commun. 2015, 51, 16188. |
| [12] | (d) Huang, Z.; Yang, X.; Yang, F.; Lu, T.; Zhou, Q. Org. Lett. 2017, 19, 3524. |
| [13] | (a) Cowen, B. J.; Miller, S. J. Chem. Soc. Rev. 2009, 38, 3102. |
| [13] | (b) Cardoso, A. L.; Beja, A. M.; Silva, M. R.; de los Santos, J. M.; Palacios, F.; Abreu, P. E.; Pais, A. A.C.C.; Pinho e Melo, T. M. V. D. Tetrahedron 2010, 66, 7720. |
| [13] | (c) Zhou, Q.-F.; Zhang, K.; Kwon, O. Tetrahedron Lett. 2015, 56, 3273. |
| [13] | (d) Wang, T.; Hoon, D. L.; Lu, Y. Chem. Commun. 2015, 51, 10186. |
| [13] | (e) Chen, Z.; Zhu, G.; Jiang, Q.; Xiao, D.; Cao, P.; Zhang, X. J. Org. Chem. 1998, 63, 5631. |
| [13] | (f) Smith, S. W.; Fu, G. C. J. Am. Chem. Soc. 2009, 131, 14231. |
| [13] | (g) Sun, J.; Fu, G. C. J. Am. Chem. Soc. 2010, 132, 4568. |
| [14] | (a) Meng, X.; Huang, Y.; Zhao, H.; Xie, P.; Ma, J.; Chen, R. Org. Lett. 2009, 11, 991. |
| [14] | (b) Xing, J.; Lei, Y.; Gao, Y.-N.; Shi, M. Org. Lett. 2017, 19, 2382. |
| [14] | (c) Wu, L.; Chen, K.; Huang, Y.; Li, E. Asian J. Org. Chem. 2020, 9, 1179. |
| [14] | (d) Debnath, S.; Kumar, A. S.; Chauhan, S.; Kumara, S. K. C. J. Org. Chem. 2021, 86, 11583. |
| [15] | (a) Ye, L.-W.; Zhou, J.; Tang, Y. Chem. Soc. Rev. 2008, 37, 1140. |
| [15] | (b) Li, E.; Huang, Y. Chem. Commun. 2014, 50, 948. |
| [15] | (c) Li, N.; Jia, P.; Huang, Y. Chem. Commun. 2019, 55, 10976. |
| [16] | Hong, L.; Wang, R. Adv. Synth. Catal. 2013, 355, 1023. |
| [17] | Tang, X.; Ni, H.; Lu, Y. Org. Chem. Front. 2021, 8, 4485. |
| [18] | Zhang, J.-Q.; Li, S.-M.; Wu, C.-F.; Wang, X.-L.; Wu, T.-T.; Du, Y.; Yang, Y.-Y.; Fan, L.-L.; Dong, Y.-X.; Wang, J.-T.; Tang, L. Catal. Commun. 2020, 138, 105838. |
| [19] | Cao, Z.; Wang, Y.; Kalita, S. J.; Schneider, U.; Huang, Y. Angew. Chem., Int. Ed. 2020, 59, 1884. |
| [20] | Wang, Y.-H.; Zhao, Z.-N.; Kalita, S. J.; Huang, Y.-Y. Org. Lett. 2021, 23, 8147. |
| [21] | Pang, S.; Yang, X.; Cao, Z.-H.; Zhang, Y.-L.; Zhao, Y.; Huang, Y.-Y. ACS Catal. 2018, 8, 5193. |
| [22] | (a) Xu, X.-H.; Wang, X.; Liu, G.; Tokunaga, E.; Shibata, N. Org. Lett. 2012, 14, 2544. |
| [22] | (b) Qiu, B.; Xu, D.; Sun, Q.; Lin, J.; Sun, W. Org. Lett. 2019, 21, 618. |
/
| 〈 |
|
〉 |