

过渡金属催化不对称环化反应合成七元环化合物研究进展
收稿日期: 2022-02-06
修回日期: 2022-03-23
网络出版日期: 2022-04-11
基金资助
国家自然科学基金(22071113); 天津市重点研发计划(19YFZCSN00240)
Research Progress on the Asymmetric Cyclization Synthesis of Seven-Membered Rings via Transition Metal Catalysis
Received date: 2022-02-06
Revised date: 2022-03-23
Online published: 2022-04-11
Supported by
National Natural Science Foundation of China(22071113); Key Technologies R & D Program of Tianjin City(19YFZCSN00240)
毛沅浩 , 高延峰 , 苗志伟 . 过渡金属催化不对称环化反应合成七元环化合物研究进展[J]. 有机化学, 2022 , 42(7) : 1904 -1924 . DOI: 10.6023/cjoc202202005
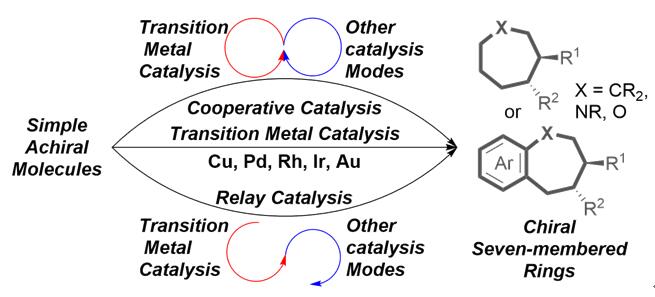
Seven-membered ring skeletons widely exist in many natural products and drug molecules, and it is of great significance to develop efficient asymmetric synthesis methods for seven-membered ring compounds. The progress in the asymmetric synthesis of seven-membered ring compounds by transition metal-catalyzed cycloaddition reactions since 2000 is reviewed. The future development direction of this field is also prospected.
| [1] | (a) Watthey, J. W. H.; Stanton, J. L.; Desai, M.; Babiarz, J. E.; Finn, B. M. J. Med. Chem. 1985, 28, 1511. |
| [1] | (b) McDonald, L. A.; Abbanat, D. R.; Barbieri, L. R.; Bernan, V. S.; Discafani, C. M.; Greenstein, M.; Janota, K.; Korshalla, J. D.; Lassota, P.; Tischler, M.; Carter, G. T. Tetrahedron Lett. 1999, 40, 2489. |
| [1] | (c) Donnell, A. F.; Michoud, C.; Rupert, K. C.; Han, X.; Aguilar, D.; Frank, K. B.; Fretland, A. J.; Gao, L.; Goggin, B.; Hogg, J. H.; Hong, K.; Janson, C. A.; Kester, R. F.; Kong, N.; Le, K.; Li, S.; Liang, W.; Lombardo, L. J.; Lou, Y.; Lukacs, C. M.; Mischke, S.; Moliterni, J. A.; Polonskaia, A.; Schutt, A. D.; Solis, D. S.; Specian, A.; Taylor, R. T.; Weisel, M.; Remiszewski, S. W. J. Med. Chem. 2013, 56, 7772. |
| [1] | (d) Xu, J. B.; Zhang, H.; Gan, L. S.; Han, Y. S.; Wainberg, M. A.; Yue, J. M. J. Am. Chem. Soc. 2014, 136, 7631. |
| [1] | (e) Ni, G.; Zhang, H.; Fan, Y.-Y.; Liu, H. C.; Ding, J.; Yue, J. M. Org. Lett., 2016, 18, 1880. |
| [1] | (f) Ruan, Q. F.; Jiang, S. Q.; Zheng, X. Y.; Tang, Y. Q.; Yang, B.; Yi, T.; Jin, J.; Cui, H.; Zhao, Z. Chem. Commun. 2020, 56, 1517. |
| [2] | (a) Carruthers, W. Cycloaddition Reactions in Organic Synthesis, Pergamon, Oxford, 1990, Vol. 373. |
| [2] | (b) Ghosez, L.; Trost, B. M. Stereocontrolled Organic Synthesis, Blackwell Science, Oxford, 1994, pp. 193-233. |
| [2] | (c) Curran, D. P. Advances in Cycloaddition, JAI Press, Greenwich, 1994, Vol. 1-3. |
| [2] | (d) Nishiwaki, N. Methods and Applications of Cycloaddition Reactions in Organic Syntheses, Wiley, Hoboken, 2014. |
| [3] | (a) Denmark, S. E.; Thorarensen, A. Chem. Rev. 1996, 96, 137. |
| [3] | (b) Chopade, P. R.; Louie, J. Adv. Synth. Catal. 2006, 348, 2307. |
| [3] | (c) Alcaide, B.; Almendros, P.; Aragoncillo, C. Chem. Soc. Rev. 2010, 39, 783. |
| [3] | (d) Ljpez, F.; MascareÇas, J. L. Chem. Soc. Rev. 2014, 43, 2904. |
| [3] | (e) Poplata, S.; Trçster, A.; Zou, Y.; Bach, T. Chem. Rev. 2016, 116, 9748. |
| [3] | (f) Pellissier, H. Synthesis 2020, 52, 3837. |
| [3] | (g) Trost, B. M.; Mata, G. Acc. Chem. Res. 2020, 53, 1293. |
| [3] | (h) Yang, C.; Yang, Z.-X.; Ding, C.-H.; Xu, B.; Hou, X. L. Chem. Rec. 2021, 21, 1442. |
| [4] | (a) Battiste, M. A.; Pelphrey, P. M.; Wright, D. L. Chem.-Eur. J. 2006, 12, 3438. |
| [4] | (b) Butenschçn, H. Angew. Chem., Int. Ed. 2008, 47, 5287. |
| [4] | (c) Harmata, M. Chem. Commun. 2010, 46, 8886. |
| [5] | (a) Trost, B. M.; Zuo, Z.; Schultz, J. E. Chem.-Eur. J. 2020, 26, 15354. |
| [5] | (b) Saranya, P. V.; Neetha, M.; Radhika, S.; Anilkumar, G. J. Heterocycl. Chem. 2021, 58, 673. |
| [6] | Hu, J.-L.; Wang, L.; Xu, H.; Xie, Z.; Tang, Y. Org. Lett. 2015, 17, 2680. |
| [7] | Wei, L.; Wang, Z.-F.; Yao, L.; Qiu, G.; Tao, H.; Li, H.; Wang, C. J. Adv. Synth. Catal. 2016, 358, 3955. |
| [8] | Wei, L.; Yao, L.; Wang, Z.-F.; Li, H.; Tao, H.-Y.; Wang, C. J. Adv. Synth. Catal. 2016, 358, 3748. |
| [9] | Wang, Y.; Zhu, L.; Wang, M.; Xiong, J.; Chen, N.; Feng, X.; Xu, Z.; Jiang, X. Org. Lett. 2018, 20, 6506. |
| [10] | Zhang, Z. J.; Zhang, L.; Geng, R. L.; Song, J.; Chen, X. H.; Gong, L. Z. Angew. Chem., Int. Ed. 2019, 58, 12190. |
| [11] | Zhu, X. Q.; Wang, Z. S.; Hou, B. S.; Zhang, H. W.; Deng, C.; Ye, L. W. Angew. Chem., Int. Ed. 2020, 59, 1666. |
| [12] | (a) Alexakis, A.; Krause, N.; Woodward, S. Copper-Catalyzed Asymmetric Synthesis, Wiley, Hoboken, 2014, pp. 1-2. |
| [12] | (b) Enthaler, S.; Wu, X. F. Zinc Catalysis Wiley, Hoboken, 2015, pp. 1-4. |
| [13] | Gulías, M.; Durán, J.; López, F.; Castedo, L.; Mascareñas, J. L. J. Am. Chem. Soc. 2007, 129, 11026. |
| [14] | Verdugo, F.; Villarino, L.; Durán, J.; Gulías, M.; Mascareñas, J. L.; López, F. ACS Catal. 2018, 8, 6100. |
| [15] | Shintani, R.; Murakami, M.; Tsuji, T.; Tanno, H.; Hayashi, T. Org. Lett. 2009, 11, 5642. |
| [16] | (a) Guo, C.; Fleige, M.; Janssen-Müller, D.; Daniliuc, C. G.; Glorius, F. J. Am. Chem. Soc. 2016, 138, 7840. |
| [16] | (b) Guo, C.; Janssen-Müller, D.; Fleige, M.; Lerchen, A.; Daniliuc, C. G.; Glorius, F. J. Am. Chem. Soc. 2017, 139, 4443. |
| [17] | Singha, S.; Patra, T.; Daniliuc, C. G.; Glorius, F. J. Am. Chem. Soc. 2018, 140, 3551. |
| [18] | Jiang, F.; Yuan, F. R.; Jin, L. W.; Mei, G. J.; Shi, F. ACS Catal. 2018, 8, 10234. |
| [19] | Cheng, Q.; Xie, J. H.; Weng, Y. C.; You, S. L. Angew. Chem., Int. Ed. 2019, 58, 5739. |
| [20] | Liu, Y. Z.; Wang, Z.; Huang, Z.; Zheng, X.; Yang, W. L.; Deng, W. P. Angew. Chem., Int. Ed. 2020, 59, 1238. |
| [21] | Trost, B. M.; Zuo, Z. Angew. Chem., Int. Ed. 2020, 59, 1243. |
| [22] | Kumari, P.; Liu, W.; Wang, C. J.; Dai, J.; Wang, M. X.; Yang, Q. Q.; Deng, Y. H.; Shao, Z. Chin. J. Chem. 2020, 38, 151. |
| [23] | Yang, W. L.; Huang, Z.; Liu, Y. Z.; Yu, X.; Deng, W. P. Chin. J. Chem. 2020, 38, 1571. |
| [24] | Wei, Y.; Liu, S.; Li, M. M.; Li, Y.; Lan, Y.; Lu, L. Q.; Xiao, W. J. J. Am. Chem. Soc. 2019, 141, 133. |
| [25] | Li, M. M.; Xiong, Q.; Qu, B. L.; Xiao, Y. Q.; Lan, Y.; Lu, L. Q.; Xiao, W. J. Angew. Chem., Int. Ed. 2020, 59, 17429. |
| [26] | Zheng, X.; Sun, H.; Yang, W. L.; Deng, W. P. Sci. China: Chem. 2020, 63, 911. |
| [27] | Liu, Y. Z.; Wang, Z.; Huang, Z.; Yang, W. L.; Deng, W. P. Org. Lett. 2021, 23, 948. |
| [28] | Gao, Y. F.; Zhang, X. G.; Zhang, X. Y.; Miao, Z. W. Org. Lett. 2021, 23, 2415. |
| [29] | Tsuji, J. Palladium Reagents and Catalysts, Wiley, Hoboken, 2004, pp. 1-26. |
| [30] | Wender, P. A.; Haustedt, L. O.; Lim, J.; Love, J. A.; Williams, T. J.; Yoon, J. Y. J. Am. Chem. Soc. 2006, 128, 6302. |
| [31] | Shintani, R.; Nakatsu, H.; Takatsu, K.; Hayashi, T. Chem.-Eur. J. 2009, 15, 8692. |
| [32] | Feng, J. J.; Lin, T. Y.; Wu, H. H.; Zhang, J. L. J. Am. Chem. Soc. 2015, 137, 3787. |
| [33] | Guzmán, P. E.; Lian, Y.; Davies, H. M. L. Angew. Chem., Int. Ed. 2014, 53, 13083. |
| [34] | Xu, G.; Chen, L.; Sun, J. Org. Lett. 2018, 20, 3408. |
| [35] | Jia, Z. J.; Shan, G.; Daniliuc, C. G.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2018, 57, 14493. |
| [36] | (a) Huang, L.; Dai, L.-X.; You, S.-L.. J. Am. Chem. Soc. 2016, 138, 5793. |
| [36] | (b) Huang, L.; Cai, Y.; Zheng, C.; Dai, L. X.; You, S. L. Angew. Chem., Int. Ed. 2017, 56, 10545. |
| [37] | Chen, Z. C.; Chen, Z.; Yang, Z. H.; Guo, L.; Du, W.; Chen, Y. C. Angew. Chem., Int. Ed. 2019, 58, 15021. |
| [38] | Yang, W. L.; Ni, T.; Deng, W. P. Org. Lett. 2021, 23, 588. |
| [39] | Li, Y. Y.; Li, S.; Fan, T.; Zhang, Z. J.; Song, J.; Gong, L. Z. ACS Catal. 2021, 11, 14388. |
| [40] | Alonso, I.; Faustino, H.; López, F.; Mascareñas, J. L. Angew. Chem., Int. Ed. 2011, 50, 11496. |
| [41] | Kardile, R. D.; Chao, T. H.; Cheng, M. J.; Liu, R. S. Angew. Chem., Int. Ed. 2020, 59, 10396. |
/
| 〈 |
|
〉 |