

均相催化丙二酸二甲酯加氢制1,3-丙二醇的研究
收稿日期: 2022-02-25
修回日期: 2022-04-07
网络出版日期: 2022-04-22
基金资助
国家自然科学青年基金(21802010); 及安徽建筑大学科研启动基金(2020QDZ03)
Homogeneous Catalytic Hydrogenation of Dimethyl Malonate into 1,3-Propanediol
Received date: 2022-02-25
Revised date: 2022-04-07
Online published: 2022-04-22
Supported by
National Natural Science Foundation for Young Scientists of China(21802010); Starting Grants of Anhui Jianzhu University(2020QDZ03)
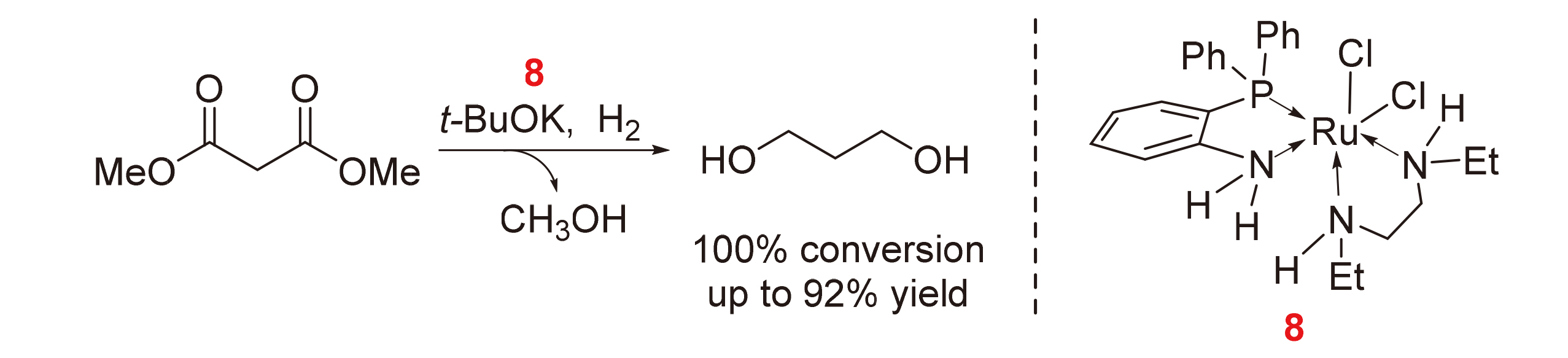
将系列o-二苯基膦苯胺配体构成的Ru(II)配合物[(PPh3)(o-PPh2C6H4NH2)RuCl2]2 (1)、(o-PPh2C6H4NHR)2RuCl2 (R=H, 2; Me, 3; Et, 4; CH2Ph, 5)和(o-PPh2C6H4NH2)[(CH2NHR)2]RuCl2 (R=H, 6; Me, 7; Et, 8; iPr, 9)应用于催化丙二酸二甲酯加氢制3-羟基丙酸甲酯或1,3-丙二醇. 围绕催化加氢性能, 系统探究了配合物结构、助剂种类及用量以及溶剂等反应条件对底物转化率和目标产物收率的影响. 研究发现, 配合物8性能最优. 同时, 配合物8在催化丙二酸二乙酯等二酯类分子加氢制醇反应中也表现出一定的催化活性.
方霄龙 , 李斌 , 金杰 , 段宁 . 均相催化丙二酸二甲酯加氢制1,3-丙二醇的研究[J]. 有机化学, 2022 , 42(5) : 1407 -1413 . DOI: 10.6023/cjoc202202034
Ruthenium complexes [(PPh3)(o-PPh2C6H4NH2)RuCl2]2 (1), (o-PPh2C6H4NHR)2RuCl2 (R=H, 2; Me, 3; Et, 4; CH2Ph, 5) and (o-PPh2C6H4NH2)[(CH2NHR)2]RuCl2 (R=H, 6; Me, 7; Et, 8; iPr, 9) were applied to the catalytic hydrogenation of dimethyl malonate into methyl 3-hydroxypropanoate or 1,3-propanediol. Targeting on the objective of the catalytic hydrogenation efficiency, the influence factors on the dimethyl malonate conversion and methyl 3-hydroxypropanoate or 1,3-propanediol selectivity, such as the structure of the ruthenium complex, the type and amount of the catalyst assistant and solvent, were well investigated. The results revealed that complex 8 resulted in the best catalytic results. Meanwhile, complex 8 could also catalyze the hydrogenation of diesters such as diethyl malonate.

| [1] | Kraus, G. A. Clean 2008, 36, 648. |
| [2] | (a) Slaugh, L. H.; Weider, P. R. US 5256827, 1993. |
| [2] | (b) Powell, J. B.; Mullin, S. B.; Weider, P. R.; Eubanks, D. C.; Arhancet, J. P. US 5770776, 1998. |
| [3] | Arntz, D.; Wiegand, N. US 5015789, 1991. |
| [4] | (a) Wang, Y.; Zhou, J.; Guo, X. RSC Adv. 2015, 5, 74611. |
| [4] | (b) Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Appl. Catal. B: Environ. 2016, 193, 75. |
| [4] | (c) Tong, Q.; Zong, A.; Gong, W.; Yu, L.; Fan, Y. RSC Adv. 2016, 6, 86663. |
| [4] | (d) Tong, Q.; Gao, Q.; Xu, B. L.; Yu, L.; Fan, Y. N. Chin. J. Org. Chem. 2017, 37, 753. (in Chinese) |
| [4] | (仝庆, 高强, 许波连, 俞磊, 范以宁, 有机化学, 2017, 37, 753.) |
| [5] | (a) Kaur, G.; Srivastava, A. K.; Chand, S. Biochem. Eng. J. 2012, 64, 106. |
| [5] | (b) Lee, C. S.; Aroua, M. K.; Daud, W. M. A. W.; Cognet, P.; Pérès-Lucchese, Y.; Fabre, P. L.; Reynes, O.; Latapie, L. Renewable Sustainable Energy Rev. 2015, 42, 963. |
| [6] | He, L.; Gong, X.; Ye, L.; Duan, X.; Yuan, Y. J. Energy Chem. 2016, 25, 1038. |
| [7] | (a) Ding, T.; Tian, H.; Liu, J.; Wu, W.; Zhao, B. Catal. Commun. 2016, 74, 10. |
| [7] | (b) Ding, T.; Tian, H.; Liu, J.; Wu, W.; Yu, J. Chin. J. Catal. 2016, 37, 484. |
| [7] | (c) Zheng, S.; Zhu, K.; Li, W.; Ji, Y. New J. Chem. 2017, 41, 5752. |
| [7] | (d) Yu, J.; Cao, J.; Du, L.; Wei, Y.; Wang, T.; Tian, H. Appl. Catal. A: Gen. 2018, 555, 161. |
| [7] | (e) Li, J.; Wan, T.; Zhang, J. J.; Fu, Y. Chin. J. Org. Chem. 2019, 39, 3258. (in Chinese) |
| [7] | (李江, 万童, 张俊杰, 傅尧, 有机化学, 2019, 39, 3258.) |
| [8] | (a) Ziebart, C.; Jackstell, R.; Beller, M. ChemCatChem 2013, 5, 3228. |
| [8] | (b) Li, W.; Xie, J. H.; Yuan, M. L.; Zhou, Q. L. Green Chem. 2014, 46, 4081. |
| [8] | (c) Wang, Y.; Wu, Z.; Li, Q.; Zhu, B.; Yu, L. Catal. Sci. Technol. 2017, 7, 3747. |
| [8] | (d) Zhang, D.; Deng, X.; Zhang, Q.; Han, J.; Yu, L. Mater. Lett. 2019, 234, 216. |
| [8] | (e) Chen, C.; Cao, Y.; Wu, X.; Cai, Y.; Liu, J.; Xu, L.; Ding, K.; Yu, L. Chin. Chem. Lett. 2020, 31, 1078. |
| [9] | Ohkuma, T.; Ooka, H.; Hashiguchi, S.; Ikariya, T.; Noyori, R. J. Am. Chem. Soc. 1995, 117, 2675. |
| [10] | (a) Zhao, B.; Han, Z.; Ding, K. Angew. Chem., Int. Ed. 2013, 52, 4744. |
| [10] | (b) Werkmeister, S.; Junge, K.; Beller, M. Org. Process Res. Dev. 2014, 18, 289. |
| [10] | (c) Pritchard, J.; Filonenko, G. A.; van Putten, R.; Hensen, E. J. M.; Pidko, E. A. Chem. Soc. Rev. 2015, 44, 3808. |
| [10] | (d) Zhou, Y.; Khan, R.; Fan, B.; Xu, L. Synthesis 2019, 51, 2491. |
| [10] | (e) Gu, G. X.; Hu, Y.; Liu, S. D.; Dong, X. Q.; Zhang, X. M. Chin. J. Org. Chem. 2020, 40, 997. (in Chinese) |
| [10] | (古国贤, 胡杨, 刘绍东, 董秀琴, 张绪穆, 有机化学, 2020, 40, 997.) |
| [10] | (f) Huang, W. B.; Qiu, L. Q.; Ren, F. Y.; He, L. N. Chin. J. Org. Chem. 2021, 41, 3914. (in Chinese) |
| [10] | (黄文斌, 邱丽琪, 任方煜, 何良年, 有机化学, 2021, 41, 3914.) |
| [11] | Fang, X. L.; Duan, N.; Zhang, M.; Zhang, C. Y.; Liu, R.; Zhu, H. P. Chin. J. Org. Chem. 2019, 39, 1450. (in Chinese) |
| [11] | (方霄龙, 段宁, 章敏, 张春燕, 刘睿, 朱红平, 有机化学, 2019, 39, 1450.) |
| [12] | (a) Fang, X.; Zhang, C.; Chen, J.; Zhu, H.; Yuan, Y. RSC Adv. 2016, 6, 45512. |
| [12] | (b) Fang, X.; Sun, M.; Zheng, J.; Li, B.; Ye, L.; Wang, X.; Cao, Z.; Zhu, H.; Yuan, Y. Sci. Rep. 2017, 7, 3961. |
| [12] | (c) Fang, X.; Li, B.; Zheng, J.; Wang, X.; Zhu, H.; Yuan, Y. Dalton Trans. 2019, 48, 2290. |
| [12] | (d) Fang, X. L.; Duan, N.; Zhang, M.; Li, B. Chin. J. Org. Chem. 2020, 40, 2692. (in Chinese) |
| [12] | (方霄龙, 段宁, 章敏, 李斌, 有机化学, 2020, 40, 2692.) |
| [13] | (a) Grey, R. A.; Pez, G. P.; Wallo, A. J. Am. Chem. Soc. 1981, 103, 7536. |
| [13] | (b) Balaraman, E.; Fogler, E.; Milstein, D. Chem. Commun. 2012, 48, 1111. |
| [14] | (a) Abdur-Rashid, K.; Guo, R.; Lough, A. J.; Morris, R. H.; Song, D. Adv. Synth. Catal. 2005, 347, 571. |
| [14] | (b) Saudan, L. A.; Saudan, C. M.; Debieux, C.; Wyss, P. Angew. Chem., Int. Ed. 2007, 46, 7473. |
| [14] | (c) Jia, W.; Chen, X.; Guo, R.; Sui-Seng, C.; Amoroso, D.; Lough, A. J.; Abdur-Rashid, K. Dalton Trans. 2009, 8301. |
| [15] | Lv, Z. G.; Wang, H. S.; Guo, Z. M.; Ge, X. P. J. Mol. Catal. 2010, 32, 417. (in Chinese) |
| [15] | (吕志果, 王恒生, 郭振美, 葛晓萍, 分子催化, 2010, 32, 417.) |
| [16] | (a) Han, Z.; Rong, L.; Wu, J.; Zhang, L.; Wang, Z.; Ding, K. Angew. Chem., Int. Ed. 2012, 51, 13041. |
| [16] | (b) Tan, X.; Wang, Y.; Liu, Y.; Wang, F.; Shi, L.; Lee, K.-H.; Lin, Z.; Lv, H.; Zhang, X. Org. Lett. 2015, 17, 454. |
| [17] | (a) Herd, O.; Heßler, A.; Hingst, M.; Tepper, M.; Stelzer, O. J. Organomet. Chem. 1996, 522, 69. |
| [17] | (b) Gao, J. X.; Wan, H. L.; Kwok, W. W.; Tse, M. C.; Wong, W. T. Polyhedron 1996, 15, 1241. |
| [17] | (c) Hingst, M.; Tepper, M.; Stelzer, O. Eur. J. Inorg. Chem. 1998, 1998, 73. |
| [17] | (d) Habtemariam, A.; Watchman, B.; Potter, B. S.; Palmer, R.; Parsons, S.; Parkin, A.; Sadler, P. J. J. Chem. Soc., Dalton Trans. 2001, 1306. |
| [17] | (e) Ossola, F.; Tisato, F.; Refosco, F. Inorg. Chim. Acta 2002, 330, 17. |
| [17] | (f) Wang, H. Y.; Jin, G. X. Eur. J. Inorg. Chem. 2005, 2005, 1665. |
| [17] | (g) Wang, D.; Li, S.; Liu, X.; Gao, W.; Cui, D. Organometallics 2008, 27, 6531. |
/
| 〈 |
|
〉 |