

苦楝甾醇及2α,3α,20R-三羟基孕甾-16β-甲基丙烯酸酯的合成
收稿日期: 2022-03-21
修回日期: 2022-04-27
网络出版日期: 2022-05-07
Synthesis of Azedarachol and 2α,3α,20R-Trihydroxypregnane-16β-methacrylate
Received date: 2022-03-21
Revised date: 2022-04-27
Online published: 2022-05-07
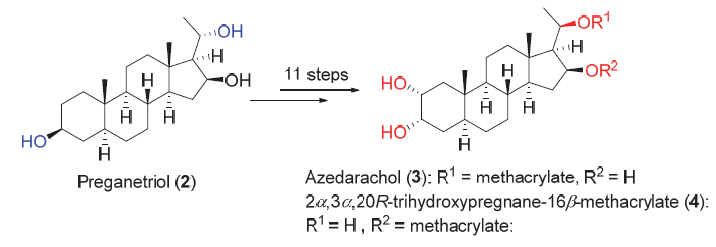
苦楝甾醇(3)及2α,3α,20R-三羟基孕甾-16β-甲基丙烯酸酯(4)都分离自苦楝根皮中, 苦楝甾醇具有良好的驱虫效果, 4则表现出一定抗肿瘤活性. 以孕甾三醇为原料, 发展了一条昆虫拒食剂苦楝甾醇等天然产物的合成路线. 合成路线以关键的分子间SN2反应立体专一性构建C(20)位构型, 并通过X射线证实C(20)位绝对构型, 以底物控制的C(23)双键双羟化反应构筑C(23)-α-双羟基, 总计11步完成苦楝甾醇及2α,3α,20R-三羟基孕甾-16β-甲基丙烯酸酯的合成, 总收率分别为6%和7.5%.
关键词: 孕甾三醇; 苦楝甾醇; 2α,3α,20R-三羟基孕甾-16β-甲基丙烯酸酯; 全合成; 天然产物
高冉 , 田伟生 . 苦楝甾醇及2α,3α,20R-三羟基孕甾-16β-甲基丙烯酸酯的合成[J]. 有机化学, 2022 , 42(8) : 2521 -2526 . DOI: 10.6023/cjoc202203040
Azedarachol (3) and 2α,3α,20R-trihydroxypregnane-16β-methacrylate (4) were isolated from the root bark of Melia azedarach L. in 1984 by Nakatani and coworkers. Azedarachol (3) shows excellent antifeedant activity against the larvae of Ajrotis sejetum Denis at the concentration of 500 μg/mL, and 2α,3α,20R-trihydroxypregnane-16β-methacrylate (4) inhibits the HGC27 tumor cells with an IC50 value of (11.3±0.5) μg/mL. The noteworthy activities and the high oxidation state structure as well as the scarce of sample suppling in nature resources have attracted considerable attention from the synthetic community. Herein the synthesis of these two natural products from preganetriol (2) is reported. The route features a stereoselective inversion at C(20) and a substrate controlled dihydroxylation of C(23) double bond. 3 and 4 were obtained in an overall yields of 6% (11 steps) and 7.5% (11 steps), respectively.

| [1] | Nakatani, M.; Takao, H.; Miura, I.; Hase, T. Phytochemirtry 1985, 24, 1945. |
| [2] | (a) Wu, S. B.; Ji, Y. P.; Zhu, J. J.; Zhao, Y.; Xia, G.; Hu, Y. H.; Hu, J. F. Steroids 2009, 74, 761. |
| [2] | (b) Wu, S.-B.; Bao, Q-Y.; Wang, W.-X.; Zhao, Y.; Xia, G.; Zhao, Z.; Zeng, H.-Q.; Hu, J.-F. Planta Med. 2011, 77, 922. |
| [3] | (a) Tian, W. S.; Shi, Y. Prog. Chem. 2010, 22, 537. (in Chinese) |
| [3] | (田伟生, 史勇, 化学进展, 2010, 22, 537.) |
| [3] | (b) Tian, W. S. CN 1146457, 1996[Chem. Abstr. 1998, 128, 167599]. |
| [3] | (c) Tian, W. S. Chin. J. Org. Chem. 2000, 18, 11. (in Chinese) |
| [3] | (田伟生, 有机化学, 2000, 18, 11.) |
| [3] | (d) Tian, W. S.; Liu, S. S.; Qiu, B. K.; Wu, X. J. CN 1475494, 2004 [Chem. Abstr. 2004, 142, 374022]. |
| [3] | (e) Gui, J. H.; Wang, D. H.; Tian, W. S. Angew. Chem., nt. Ed. 2011, 50, 7093. |
| [3] | (f) Shi, Y.; Jia, L. Q.; Xiao, Q.; Lan, Q.; Tang, X. H.; Wang, D. H.; Li, M.; Ji, Y.; Zhou, T.; Tian, W. S. Chem.-Asian J. 2011, 6, 786. |
| [3] | (g) Wang, S. S.; Shi, Y.; Tian, W. S. Org. Lett. 2014, 16, 2177. |
| [4] | (a) Zhang, D. S.; Shi, Y.; Tian, W. S. Chin. J. Chem. 2015, 33, 669. |
| [5] | (a) Han, J.; Lin, J. R.; Jin, R. H.; Tian, W. S. Acta Chim. Sinica 2011, 69, 2272. (in Chinese) |
| [5] | (韩军, 林静容, 金荣华, 田伟生, 化学学报, 2011, 69, 2272.) |
| [5] | (b) Han, J. M.S Thesis, Shanghai Normal University, Shanghai, 2011. (in Chinese) |
| [5] | (韩军, 硕士论文, 上海师范大学, 上海, 2011.) |
| [6] | Compound 10/11 cannot be separated by column chromatography, and the proportion of oxidation product 10a/11a obtained by further oxidation is 74%/11%. |
/
| 〈 |
|
〉 |