

邻甲酰基苯甲酸甲酯还原胺化/内酰胺化一锅法合成N-取代异吲哚-1-酮
收稿日期: 2022-02-19
修回日期: 2022-04-08
网络出版日期: 2022-05-18
基金资助
河南省科技攻关计划(212102310883); 河南省教育厅自然科学重点研究(21B150013); 济源职业技术学院自然科学重点研究(JZXY-2020-58)
One-Pot Synthesis of N-Substituted Isoindolin-1-ones via Reductive Amination/Lactamization of Methyl 2-Formylbenzoate
Received date: 2022-02-19
Revised date: 2022-04-08
Online published: 2022-05-18
Supported by
Program for Science and Technology Development of Henan Province(212102310883); Key Natural Science Research Program of the Education Department of Henan Province(21B150013); Key Natural Scientific Research Projects of Jiyuan Vocational Technical College(JZXY-2020-58)
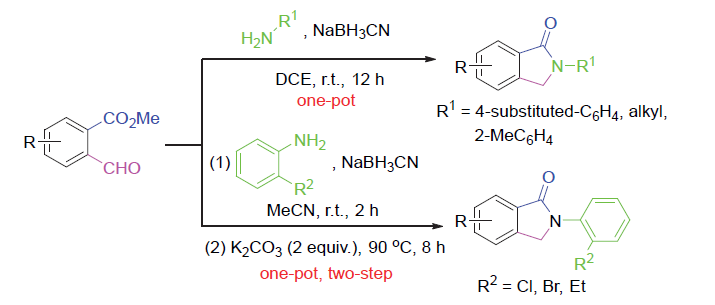
报道了一种通过邻醛基苯甲酸甲酯的胺化还原/内酰胺化一锅法合成N-取代异吲哚-1-酮的高效方法. 反应包括以NaBH3CN为还原剂, 邻醛基苯甲酸甲酯与伯芳基胺或烷基胺的胺化还原以及N原子对羰基碳的分子内亲核进攻过程. 对于4-取代芳胺、烷基胺和邻甲基苯胺, 该反应可以在室温下的二氯乙烷中顺利完成转化. 而其他的2-取代芳胺, 则需K2CO3 (2 equiv.)促进下在乙腈中回流8 h完成. 该反应的底物适用范围很广, 最高收率可达99%.
关键词: N-取代异吲哚-1-酮; 胺化还原; 内酰胺化; 一锅法合成
张文生 , 李焱 , 崔海燕 , 苏小莉 , 徐素鹏 . 邻甲酰基苯甲酸甲酯还原胺化/内酰胺化一锅法合成N-取代异吲哚-1-酮[J]. 有机化学, 2022 , 42(8) : 2456 -2461 . DOI: 10.6023/cjoc202202022
An efficient, one-pot synthesis of N-substituted isoindolin-1-ones by an aminative reduction/lactamisation sequence of methyl 2-formylbenzoate is introduced. The reaction process includes aminative reduction of o-phthalaldehydic acid methylesters with primary aryl or alkyl amines using NaBH3CN and the following intramolecular nucleophilic N-attack on carbonyl carbon atom. For 4-substituted aromatic amines, alkyl amines and o-methylaniline, the transformation can be successfully completed in 1,2-dichloroethane at room temperature. For other 2-substituted aromatic amines, reflux in acetonitrile for 8 h in the presence of K2CO3 (2 equiv.) is needed. The reaction scopes were quite broad and up to 96% yield of diverse products was achieved.

| [1] | Uno, M.; Ban, H. S.; Nakamura, H. Bioorg. Med. Chem. Lett. 2009, 19, 3166. |
| [2] | Ghosh, U.; Bhattacharyya, R.; Keche, A. Tetrahedron 2010, 66, 2148. |
| [3] | Ruchelman, A. L.; Man, H. W.; Zhang, W.; Chen, R.; Capone, L.; Kang, J.; Parton, A.; Corral, L.; Schafer, P. H.; Babusis, D.; Moghaddam, M. F.; Tang, Y.; Shirley, M. A.; Muller, G. W. Bioorg. Med. Chem. Lett. 2013, 23, 360. |
| [4] | Breytenbach, J. C.; van Dyk, S.; van den Heever, I.; Allin, S. M.; Hodkinson, C. C.; North, C. J.; Page, M. I. Bioorg. Med. Chem. Lett. 2000, 10, 1629. |
| [5] | Mark, D. J. M.; Norman, H.; Rigdon, G. C. J. Med. Chem. 1996, 39, 149. |
| [6] | Hardcastle, I. R.; Ahmed, S. U.; Atkins, H.; Calvert, A. H.; Curtin, N. J.; Farnie, G.; Golding, B. T.; Griffin, R. J.; Guyenne, S.; Hutton, C.; Kallblad, P.; Kemp, S. J.; Kitching, M. S.; Newell, D. R.; Norbedo, S.; Northen, J. S.; Reid, R. J.; Saravanan, K.; Willems, H. M.; Lunec, J. Bioorg. Med. Chem. Lett. 2005, 15, 1515. |
| [7] | Zhao, X.-Z.; Semenova, E. A.; Vu, B. C.; Maddali, K.; Marchand, C.; Hughes, S. H.; Pommier, Y.; Burke, T. R. J. Med. Chem. 2008, 51, 251. |
| [8] | Suven Das, R. F.; Pramanik, A. Org. Lett. 2006, 8, 4263. |
| [9] | (a) Pathare, R. S.; Sharma, S.; Elagandhula, S.; Saini, V.; Sawant, D. M.; Yadav, M.; Sharon, A.; Khan, S.; Pardasani, R. T. Eur. J. Org. Chem. 2016, 5579. |
| [9] | (b) Zhu, C.-L.; Zhang, J.-T.; Hoye, T. R. Org. Lett. 2021, 23, 7550. |
| [9] | (c) Hu, X.-Q.; Liu, Z.-K.; Hou, Y.-X.; Xu, J.-H.; Gao, Y. Org. Lett. 2021, 23, 6332. |
| [9] | (d) Wan, J.-P.; Wu, B.; Pan, Y.-J. Tetrahedron 2007, 63, 9338. |
| [10] | Mamidyala, S. K.; Cooper, M. A. Chem. Commun. 2013, 49, 8407. |
/
| 〈 |
|
〉 |