

三氟甲磺酸酐介导炔酰胺与吡啶加成反应的研究
收稿日期: 2022-03-31
修回日期: 2022-04-29
网络出版日期: 2022-05-27
基金资助
国家自然科学基金(21772027)
Trifluoromethyl Sulfonic Anhydride Mediated Addition of Pyridine with Ynamides
Received date: 2022-03-31
Revised date: 2022-04-29
Online published: 2022-05-27
Supported by
National Natural Science Foundation of China(21772027)
宋昊儒 , 孙建婷 , 吕敏 , 刘艺雯 , 魏邦国 . 三氟甲磺酸酐介导炔酰胺与吡啶加成反应的研究[J]. 有机化学, 2022 , 42(8) : 2433 -2437 . DOI: 10.6023/cjoc202203061
A convenient approach to pyridine quaternary ammonium salts containing enamine fragment has been developed, which features a trifluoromethyl sulfonic anhydride mediated regioselective addition process of pyridine with ynamides. Using this method, a variety of substituted pyridine quaternary ammonium salts containing enamine fragment 3a~3m were prepared in moderate to excellent yields with high regioselectivities (Z:E up to 20:1).
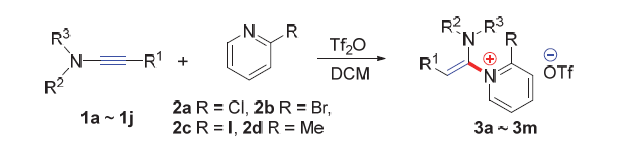
Key words: ynamides; pyridine; addition reaction; pyridine quaternary ammonium salt
| [1] | For selected see: (a) Prabagar, B.; Ghosh, N.; Sahoo, A. K. Synlett 2017, 28, 2539. |
| [1] | (b) Zhang, X.-J.; Zhang, Y.-S.; Huang, J.; Hsung, R. P.; Kurtz, K. C. M.; Oppenheimer, J.; Petersen, M. E.; Sagamanova, I. K.; Shen, L.-C.; Tracey, M. R. J. Org. Chem. 2006, 71, 4170. |
| [1] | (c) Coste, A.; Karthikeyan, G.; Couty, F.; Evano, G. Angew. Chem., Int. Ed. 2009, 48, 4381. |
| [1] | (d) Zhang, Y.-S.; Hsung, R. P.; Tracey, M. R.; Kurtz, K. C. M.; Vera, E. L. Org. Lett. 2004, 6; 1151. |
| [1] | (e) Alcaide, B.; Almendros, P.; Lázaro-Milla, C. Chem.-Eur. J. 2016, 22, 8998. |
| [1] | (f) Zhao, Y.-L.; Tu, Y.-L.; Cai, M.-Z.; Zhao, J.-F. Chin. J. Org. Chem. 2022, 42, 85. (in Chinese) |
| [1] | (赵永丽, 涂永良, 蔡明中, 赵军锋, 有机化学, 2022, 42, 85.) |
| [2] | (a) Jouvin, K.; Heimburger, J.; Evano, G. Chem. Sci. 2012, 3, 756. |
| [2] | (b) Evano, G.; Coste, A.; Jouvin, K. Angew. Chem., Int. Ed. 2010, 49, 2840. |
| [2] | (c) DeKorver, K. A.; Li, H.-Y.; Lohse, A. G.; Hayashi, R.; Lu, Z.-J.; Zhang, Y.; Hsung, R. P. Chem. Rev. 2010, 110, 5064. |
| [3] | (a) Hong, F.-L.; Wang, Z.-S.; Wei, D.-D.; Zhai, T.-Y.; Deng, G.-C.; Lu, X.; Liu, R.-S.; Ye, L.-W. J. Am. Chem. Soc. 2019, 141, 16961, |
| [3] | (b) Xu, Y.; Sun, Q.; Tan, T.-D.; Yang, M.-Y.; Yuan, P.; Wu, S.-Q.; Lu, X.; Hong, X.; Ye, L.-W. Angew. Chem., Int. Ed. 2019, 58, 16252. |
| [4] | (a) Hong, F.-L.; Ye, L.-W. Acc. Chem. Res. 2020, 53, 2003. |
| [4] | (b) Duret, G.; Le Fouler, V.; Bisseret, P.; Bizet, V.; Blanchard, N. Eur. J. Org. Chem. 2017, 2017, 6816. |
| [5] | (a) Chen, Y.-B.; Qian, P.-C.; Ye, L.-W. Chem. Soc. Rev. 2020, 49, 8897. |
| [5] | (b) Hu, Y.-C.; Zhao, Y.-Y.; Wan, B.-S.; Chen, Q.-A. Chem. Soc. Rev. 2021, 50, 2582. |
| [5] | (c) Lynch, C. C.; Sripada, A.; Wolf, C. Chem. Soc. Rev. 2020, 49, 8543. |
| [6] | Wang, C.-M.; Qi, L.-J. Sun, Q.; Zhou, B.; Shi, Z.-F.; Lin, S. C.; Lu, X.; Gong, L.; Ye, L.-W. Green Chem. 2018, 20, 3271. |
| [7] | Xi, Y.; Zhu, G.-H.; Tang, L.-N.; Ma, S.-H.; Zhang, D.-M.; Zhang, R.; He, G.-K.; Zhu, H.-J. Org. Biomol. Chem. 2017, 15, 7218. |
| [8] | Kong, Y.; Jiang, K.; Cao, J.; Fu, L.; Yu, Lai, G.-Q.; Cui, Y.-M.; Hu, Z.-Q.; Wang, G.-H. Org. Lett. 2013, 15, 422. |
| [9] | (a) Shandilya, S.; Protim Gogoi, M.; Dutta, S.; Sahoo, A. K. Chem. Rec. 2021, 21, 4123. |
| [9] | (b) Zhou, B.; Tan, T.-D.; Zhu, X.-Q.; Shang, M.-Z.; Ye, L.-W. ACS Catal. 2019, 9, 6393. |
| [9] | (c) Wang, X.-N.; Yeom, H.-S.; Fang, L.-C.; He, S.-Z.; Ma, Z.-X. Acc. Chem. Res., 2014, 47, 560. |
| [9] | (d) Meng, T.-J.; Chen, R.-X.; Liu, L.-T.; Wang, T.; Liu, X.-M.; Zhao, W.-X. Chin. J. Org. Chem. 2015, 35, 2108. (in Chinese) |
| [9] | (孟团结, 陈荣祥, 刘澜涛, 王涛, 刘新明, 赵文献, 有机化学, 2015, 35, 2108.) |
| [9] | (e) Zhu, J.-R.; Ren, X.-J.; Tang, F.-Y.; Pan, F.; Ye, L.-W. Chin. J. Org. Chem. 2019, 39, 1102. (in Chinese) |
| [9] | (朱建荣, 任小娟, 唐飞宇, 潘飞, 叶龙武, 有机化学, 2019, 39, 1102.) |
| [9] | (f) Zhu, C.-Z.; Feng, J.-J.; Zhang, J.-L. Chin. J. Org. Chem. 2017, 37, 1165. (in Chinese) |
| [9] | (朱超泽, 冯见君, 张俊良, 有机化学, 2017, 37, 1165.) |
| [10] | (a) Nie, X.-D.; Mao, Z.-Y.; Guo, J.-M.; Si, C.-M.; Wei, B.-G.; Lin, G.-Q. J. Org. Chem. 2022, 87, 2380. |
| [10] | (b) Nie, X.-D.; Han, X.-L.; Sun, J.-T.; Si, C.-M.; Wei, B.-G.; Lin, G.-Q. J. Org. Chem. 2021, 86, 3433, |
| [10] | (c) Ma, R.-J.; Xu, W.-K.; Sun, J.-T.; Chen, L.; Si, C.-M.; Wei, B.-G. Tetrahedron Lett. 2021, 67, 152873. |
| [10] | (d) Han, X.-L.; Nie, X.-D.; Feng, Y.-M.; Wei, B.-G.; Si, C.-M.; Lin, G.-Q. Chin. Chem. Lett. 2021, 32, 3526. |
| [10] | (e) Han, P.; Mao, Z.-Y.; Li, M.; Si, C.-M.; Wei, B.-G.; Lin, G.-Q. J. Org. Chem. 2020, 85, 4740. |
| [10] | (f) Han, X.-L.; Nie, X.-D.; Chen, Z.-D.; Si, C.-M.; Wei, B.-G.; Lin, G.-Q. J. Org. Chem. 2020, 85, 13567. |
| [10] | (g) Zhang, Y.-X.; Chen, L.-Y.; Sun, J.-T.; Si, C.-M.; Wei, B.-G. J. Org. Chem. 2020, 85, 12603. |
| [10] | (h) Liu, Y.-W.; Ma, R.-J.; Wang, Q.-E.; Si, C.-M.; Wei, B.-G. Tetrahedron 2020, 76, 131649. |
| [10] | (i) Han, P.; Mao, Z.-Y.; Si, C.-M.; Zhou, Z.; Wei, B.-G.; Lin, G.-Q. J. Org. Chem. 2019, 84, 914. |
| [10] | (j) Liu, Y.-W.; Mao, Z.-Y.; Nie, X.-D.; Si, C.-M.; Wei, B.-G.; Lin, G.-Q. J. Org. Chem. 2019, 84, 16254. |
| [11] | Brioche, J.; Meyer, C.; Cossy, J. Org. Lett. 2015, 17, 2800. |
| [12] | Dwivedi, V.; Kumar, R.; Sharma, K.; Sridhar, B.; Reddy, M. S. ACS Omega 2017, 2, 2770. |
| [13] | Markham, J. P.; Wang, B.; Stevens, E. D.; Burris, S. C.; Deng, Y. Chem.-Eur. J. 2019, 25, 6638. |
| [14] | CCDC 2163532 (3a) contains the supplementary crystallographic data for this paper. These data are free of charge from The Cambridge Crystallographic Centre via www.ccdc.cam.ac.uk/datarequest/cif. |
| [15] | (a) Charette, A. B.; Grenon, M.; Lemire, A.; Pourashraf, M.; Martel, J. J. Am. Chem. Soc. 2001, 123, 11829. |
| [15] | (b) Charette, A. B.; Mathieu, S.; Martel, J. Org. Lett. 2005, 7, 5401. |
| [15] | (c) Peng, B.; Geerdink, D.; Fares, C.; Maulide, N. Angew. Chem., Int. Ed. 2014, 53, 5462. |
/
| 〈 |
|
〉 |