

苯炔[3+2]环加成反应构建三氟甲基取代的苯并环状亚砜亚胺衍生物及其杀棉蚜活性研究
收稿日期: 2022-03-24
修回日期: 2022-05-13
网络出版日期: 2022-06-23
基金资助
石河子大学双一流(SHYL-YB201901); 石河子大学高层次人才启动(RCZK2021B08)
Constrution and Insecticidal Activities of Trifluoromethylated Benzocyclicsulfoximine Derivatives by [3+2] Cycloaddition Reaction of Beznyne
Received date: 2022-03-24
Revised date: 2022-05-13
Online published: 2022-06-23
Supported by
Double First Class Project of Shihezi University(SHYL-YB201901); High Level Talents Launch Project of Shehezi University(RCZK2021B08)
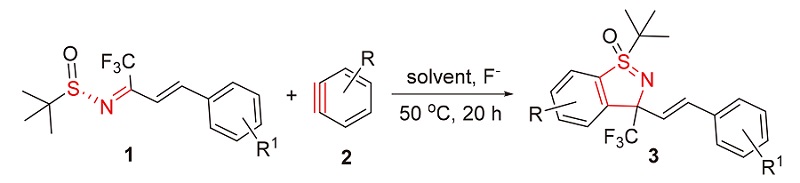
亚砜亚胺由于其抗疟、抗菌及除草等特性在农药和医药领域有广泛的应用. 以苯炔与三氟甲基取代的α,β-不饱和叔丁基亚磺酰胺通过[3+2]环加成反应构建了三氟甲基芳基取代的苯并环状亚砜亚胺骨架. 反应以氟化钾为氟试剂, 18-冠-6为添加剂, 乙腈/甲苯(V∶V=4∶1)为溶剂, 室温反应20 h, 条件温和, 收率低到中等(13%~57%). 采用浸渍法测定该类化合物对棉蚜的杀虫活性, 结果表明, (1R,3S)-1-(叔丁基)-3-((E)-4-甲基苯乙烯基)-3-(三氟甲基)苯并[d]异噻唑1-氧化物(3b)和(1R,3S)-1-(叔丁基)-3-(三氟甲基)-3-((E)-4-(三氟甲基)苯乙烯基)苯并[d]异噻唑1-氧化物(3h)的24 h LC50低于阳性对照吡虫啉(LC50 0.0800 mg/L)和啶虫脒(LC50 0.0532 mg/L), 可作为杀虫药物的先导化合物进行结构修饰研究.
李文娟 , 张睿 , 蔡志华 , 韩小强 , 何林 , 代斌 . 苯炔[3+2]环加成反应构建三氟甲基取代的苯并环状亚砜亚胺衍生物及其杀棉蚜活性研究[J]. 有机化学, 2022 , 42(9) : 2832 -2839 . DOI: 10.6023/cjoc202203051
Sulfoximines derivatives are widely used in the field of pesticides and medicine due to its antimalarial, antibacterial and herbicidal properties. A concise and direct synthetic strategy for the construction of trifluoromethylated cyclicsulfoximines has been developed via [3+2] cycloaddition reaction of benzynes and trifluoromethyl α,β-unsaturated N-(S)-tert-butyl sulfinylketoimines. The reaction affords the cyclosulfoximines at room temperature in low to moderate yields (13%~57%) and KF as fluorinated reagent, 18-C-6 as additives, and acetonitrile and toluene as mixed solvent (V∶V=4∶1). The insecticidal activities against Aphis gossypii were determined by impregnation method. The results showed that 24-h median lethal concentrations (24 h LC50) of (1R,3S)-1-(tert-butyl)-3-((E)-4-methylstyryl)-3-(trifluoromethyl)-3H-1λ4-benzo- [d]isothiazole 1-oxide) (3b) and (1R,3S)-1-(tert-butyl)-3-(trifluoromethyl)-3-((E)-4-(trifluoromethyl)styryl)-3H-1λ4-benzo[d]- isothiazole 1-oxide (3h) are lower than that of the positive control imidacolprid (0.0800 mg/L) and dinitraz (0.0532 mg/L). They can be used as new lead insecticidal compounds with further structure modification.

| [1] | (a) Himeshima, Y.; Sonoda, T.; Kobayashi, H. Chem. Lett. 1983, 12, 1211. |
| [1] | (b) For a facile two-step preparation of o-silylaryltriflates from the corresponding o-bromophenols, see: Peña, D.; Cobas, A.; Pérez, D.; Guitián, E. Synthesis 2002, 1454. |
| [2] | For selected examples of insertion-cyclization reaction of arynes, see: (a) Gilmore, C. D.; Allan, K. M.; Stoltz, B. M. J. Am. Chem. Soc. 2008, 130, 1558. |
| [2] | (b) Li, Y. Y.; Qiu, D. C.; Gu, R. R.; Wang, J. L.; Shi, J. R.; Li, Y. J. Am. Chem. Soc. 2016, 138, 10814. |
| [2] | (c) Shi, J.; Qiu, D.; Wang, J.; Xu, H.; Li, Y. J. Am. Chem. Soc. 2015, 137, 5670. |
| [2] | (d) Rao, B.; Tang, J.; Wei, Y.; Zeng, X. Chem.-Asian J. 2016, 11, 991. |
| [2] | (e) Sundalam, S. K.; Nilova, A.; Seidl, T. L.; Stuart, D. R. Angew. Chem., nt. Ed. 2016, 55, 8431. |
| [2] | (f) Thangaraj, M.; Bhojgude, S. S.; Jain, S.; Gonnade, R. G.; Biju, A. T. J. Org. Chem. 2016, 81, 8604. |
| [2] | (g) Tao, Y.; Zhang, F.; Tang, C. Y.; Wu, X. Y.; Sha, F. Asian J. Org. Chem. 2014, 3, 1292. |
| [3] | For selected examples, see: (a) Shi, F.; Waldo, J. P.; Chen, Y.; Larock, R. C. Org. Lett. 2008, 10, 2409. |
| [3] | (b) Fang, Y.; Larock, R. C.; Shi, F. Asian J. Org. Chem. 2014, 3, 55. |
| [3] | (c) Ikawa, T.; Takagi, A.; Goto, M. J. Org. Chem. 2013, 78, 2965. |
| [3] | (d) Swain, S. P.; Shih, Y. C.; Tsay, S. C. Angew. Chem., nt. Ed. 2015, 54, 9926. |
| [4] | For selected examples of multicomponent reactions of arynes, see: (a) Suh, S. E.; Chenoweth, D. M. Org. Lett. 2016, 18, 4080. |
| [4] | (b) Bhojgude, S. S.; Baviskar, D. R.; Gonnade, R. G.; Biju, A. T. Org. Lett. 2015, 17, 6270. |
| [4] | (c) Zeng, Y. W.; Li, G. Y.; Hu, J. B. Angew. Chem., nt. Ed. 2015, 4, 10773. |
| [4] | (d) Sha, F.; Huang, X. Angew. Chem., nt. Ed. 2009, 48, 3458. |
| [4] | (e) Yoshida, H.; Asatsu, Y.; Mimura, Y.; Ito, Y.; Ohshita, J.; Takaki, K. Angew. Chem., nt. Ed. 2011, 50, 9676. |
| [5] | (a) Goldberg, F. W.; Kettle, J. G.; Xiong, J.; Lin, D. Tetrahedron 2014, 70, 6613. |
| [5] | (b) Goldberg, F. W.; Kettle, J. G.; Kogej, T.; Perry, M. W. D.; Tomkinson, N. P. Drug Discovery Today 2015, 20, 11. |
| [5] | (c) Nishimura, N.; Norman, M. H.; Liu, L.; Yang, K. C.; Ashton, K. S.; Bartberger, M. D.; Chmait, S.; Chen, J.; Cupples, R.; Fotsch, C.; Helmering, J.; Jordan, S. R.; Kunz, R. K.; Pennington, L. D.; Poon, S. F.; Siegmund, A.; Sivits, G.; Lloyd, D. J.; Hale, C.; St. Jean, D. J. J. Med. Chem. 2014, 57, 3094. |
| [5] | (d) Nugent, B. M.; Buysse, A. M.; Loso, M. R.; Babcock, J. M.; Johnson, T. C.; Oliver, M. P.; Martin, T. P.; Ober, M. S.; Breaux, N.; Robinson, A.; Adelfinskaya, Y. Pest Manage. Sci. 2015, 71, 928. |
| [5] | (e) von Nussbaum, F.; Li, V. M.-J.; Allerheiligen, S.; Anlauf, S.; Bärfacker, L.; Bechem, M.; Delbeck, M.; Fitzgerald, M. F.; Gerisch, M.; Gielen-Haertwig, H.; Haning, H.; Karthaus, D.; Lang, D.; Lustig, K.; Meibom, D.; Mittendorf, J.; Rosentreter, U.; Schäfer, M.; Schäfer, S.; Schamberger, J.; Telan, L. A.; Tersteegen, A. ChemMedChem 2015, 10, 1163. |
| [5] | (f) Mendonça Matos, P.; Lewis, W.; Argent, S. P.; Moore, J. C.; Stockman, R. A. Org. Lett. 2020, 22, 2776. |
| [5] | (g) Bär, R.; Langer, L.; Nieger, M.; Bräse, S. Adv. Synth. Catal. 2020, 362, 1356. |
| [6] | Johnson, C. R.; Janiga, E. R. J. Am. Chem. Soc. 1973, 95, 7692. |
| [7] | Moragas, T.; Liffey, R. M.; Regentová, D.; Ward, J.-P. S.; Dutton, J.; Lewis, W.; Stockman, R. A. Angew. Chem., n. Ed. 2016, 55, 10047. |
| [8] | Cividino, P.; Verrier, C.; Philouze, C.; Carret, S.; Poisson, J.-F. Adv. Synth. Catal. 2019, 361, 1236. |
| [9] | Lücking, U.; Boulard, E.; Zibulski, V.; Lienau, P.; Oertel, L.; Schaefer, M.; Ganzer, U. Chem.-Eur. J. 2020, 26, 4378. |
| [10] | Aota, Y.; Maeda, Y.; Kano, T.; Maruoka, K. Chem.-Eur. J. 2019, 25, 15755. |
| [11] | Zhang, D.; Wang, H.; Cheng, H.; Hernández, J. G.; Bolm, C. Adv. Synth. Catal. 2017, 359, 4274. |
| [12] | Ye, W.; Zhang, L.; Ni, C.; Rong, J.; Hu, J. Chem. Commun. 2014, 50, 10596. |
| [13] | Shen, X.; Hu, J. Eur. J. Org. Chem. 2014, 2014, 4437. |
| [14] | (a) He, L; Pian, J. X.; Shi, J. F.; Du, G. F.; Dai, B. Tetrahedron 2014, 70, 2400. |
| [14] | (b) Pian, J. X.; He, L.; Du, G. F.; Guo, H.; Dai, B. J. Org. Chem. 2014, 79, 5820. |
| [14] | (c) Liu, K.; Liu, L. L.; Gu, C. Z.; Dai, B.; He, L. RSC Adv. 2016, 6, 33606. |
| [14] | (d) He, L.; Pian, J. X.; Zhang, J.; Li, Y. Z. Chin. Chem. Lett. 2012, 23, 1359. |
| [14] | (e) Liu, L. L.; Li, Z. J.; Gu, C. Z.; He, L.; Dai, B. J. Saudi Chem. Soc. 2017, 21, 458. |
| [14] | (f) Jian, H.; Liu, K.; Wang, W. H.; Li, Z. J.; Dai, B.; He, L. Tetrahedron Lett. 2017, 58, 1137. |
| [14] | (g) Li, Z. J.; Jian, H.; Wang, W. H.; Wang, Q.; He, L. Chin. J. Org. Chem. 2018, 38, 2045. (in Chinese) |
| [14] | (李志娟, 翦辉, 王伟华, 王强, 何林, 有机化学, 2018, 38, 2045.) |
| [14] | (h) Li, W. J.; Luo, X. S.; Guo, L.; He, L.; Dai, B.; Guo, X. H. J. Shihezi Univ. (Nat. Sci.) 2017, 35, 273. (in Chinese) |
| [14] | (李文娟, 骆学松, 郭亮, 何林, 代斌, 郭旭红, 石河子大学学报, 2017, 35, 273.) |
| [15] | (a) Liu, Z. J.; Liu, J. T. Chem. Commun. 2008, 5233. |
| [15] | (b) Sanz-Marco, A.; Blay, G.; Muñoz, M. C.; Pedro, J. R. Chem. Commun. 2015, 51, 8958. |
| [16] | Guo, T. F.; Shi, X. Y.; Gao, X. W.; Liu, X. M. J. Environ. Entomol. 2014, 36, 388. (in Chinese) |
| [16] | (郭天凤, 史雪岩, 高希武, 刘晓宁, 环境昆虫学报, 2014, 36, 388.) |
| [17] | Zhou, Y. T. M.S. Thesis, Shihezi University, 2019. (in Chinese) |
| [17] | (周月婷, 硕士论文, 石河子大学, 2019.) |
/
| 〈 |
|
〉 |