

配体调控钯催化乙烯基环状碳酰胺和异氰酸酯的差异性转化
收稿日期: 2022-05-22
修回日期: 2022-07-25
网络出版日期: 2022-08-18
基金资助
国家自然科学基金(21822103); 国家自然科学基金(21820102003); 国家自然科学基金(91956201); 高等学校学科创新引智计划(111计划); 高等学校学科创新引智计划(B17019); 湖北省自然科学基金(2017AHB047)
Ligand-Switched Pd-Catalyzed Divergent Transformations of Vinyl Cyclic Carbamates and Isocyanates
Received date: 2022-05-22
Revised date: 2022-07-25
Online published: 2022-08-18
Supported by
National Natural Science Foundation of China(21822103); National Natural Science Foundation of China(21820102003); National Natural Science Foundation of China(91956201); Program of Introducing Talents of Discipline to Universities of China (111 Program); Program of Introducing Talents of Discipline to Universities of China(B17019); Natural Science Foundation of Hubei Province(2017AHB047)
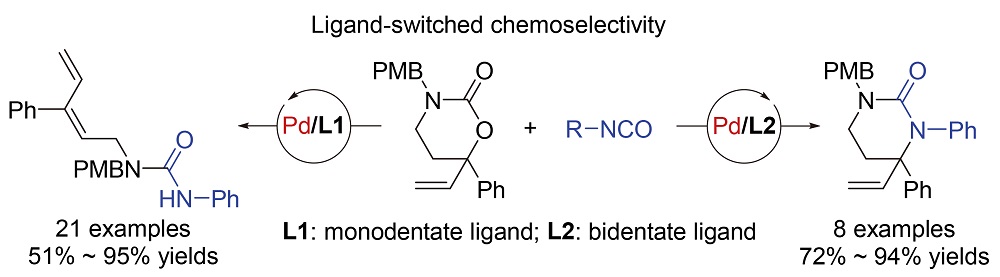
用配体调控策略, 通过钯催化发展了乙烯基环状碳酰胺和异氰酸酯之间的两类差异性转化, 选择性地合成了多取代的共轭二烯脲类化合物和四氢嘧啶酮衍生物. 当反应以Pd2(dba)3•CHCl3 (dba: dibenzylideneacetone)为催化剂前体、以单齿配位的N,N-二甲基亚磷酰胺(MonoPhos)为配体时, 可以高选择性地得到一系列线性的共轭二烯脲类衍生物; 将配体改为双齿配位的1,3-双(二苯基膦)丙烷(DPPP)时, 利用相同的原料则能够合成一系列环状的四氢嘧啶酮类化合物. 这一研究通过简便的配体调控策略, 为含氮化合物的多样性合成提供了新方法.
熊威 , 石斌 , 姜烜 , 陆良秋 , 肖文精 . 配体调控钯催化乙烯基环状碳酰胺和异氰酸酯的差异性转化[J]. 有机化学, 2023 , 43(1) : 265 -273 . DOI: 10.6023/cjoc202205038
Synthesis of urea compounds is highly significant for pharmaceutical chemistry, agricultural chemistry, matterials science and organic synthesis. By ultilizing ligands to switch the chemoselectivity, two transformations of vinyl cyclic carbamates and isocyanates were developed to afford linear and cyclic ureas in a controlled manner. When phosphoramidite was used as the monodentate P-ligand, a wide range of (E)-1,3-diene-substituted linear ureas were produced in high yields and selectivity (21 examples, 51%~95% yields). While, using bidentate biphosphine ligand to replace the phosphoramidite ligand, a variety of highly functionalized vinyl-substitued cyclic ureas, tetrahydro-2-pyrimidinones, were delivered instead starting from the same chemical feedstocks (8 examples, 72%~94% yields). To illusrate the switched chemoselectivity, two possible reaction mechanisms were proposed based on the experiment results and proceeding literatures. The keys of these two reactions, dienylation and annulation, are the β-hydride elimination and the N-allylic alkylation of ally-Pd species, respectively. It is supposed that the basicity of the ligand will affect the reactivity of π-ally-Pd intermediates toward electrophilic ring closing. It is believed that this research not only enriches the application of vinyl carbamate reagents in organic synthesis, but also provides a new protocol to access significant nitrogen-containing molecules.

Key words: ligand-switched; palladium catalysis; urea; vinyl carbamate; isocyanate
| [1] | (a) Guo, W.; Gómez, J. E.; Cristòfol, à.; Xie, J.; Kleij, A. W. Angew. Chem., Int. Ed. 2018, 57, 13735. |
| [1] | (b) Zuo, L.; Liu, T.; Chang, X.; Guo, W. Molecules 2019, 24, 3930. |
| [1] | (c) Li, T.-R.; Wang, Y.-N.; Xiao, W.-J.; Lu, L.-Q. Tetrahedron Lett. 2018, 59, 1521. |
| [2] | Knight, J. G.; Ainge, A. M.; Harwood, S. J.; Maughan, H. I.; Armour, D. R.; Hollinshead, D. M.; Jaxa-Chamiec, A. A. J. Am. Chem. Soc. 2000, 122, 2944. |
| [3] | Wang, C.; Tunge, J. A. Org. Lett. 2006, 8, 3211. |
| [4] | Ohmatsu, K.; Imagawa, N.; Ooi, T. Nat. Chem. 2014, 6, 47. |
| [5] | (a) Guo, C.; Fleige, M.; Janssen-Müller, D.; Daniliuc, C. G.; Glorious, F. J. Am. Chem. Soc. 2016, 138, 7840. |
| [5] | (b) Leth, L. A.; Glaus, F.; Meazza, M.; Fu, L.; Th?gersen, M. K.; Bitsch, E. A.; J?rgensen, K. A. Angew. Chem., Int. Ed. 2016, 55, 15272. |
| [5] | (c) Qi, Z.; Kong, L.; Li, X. Org. Lett. 2016, 18, 4392. |
| [5] | (d) Jin, J.-H.; Wang, H.; Yang, Z.-T.; Yang, W.-L.; Tang, W.; Deng, W.-P. Org. Lett. 2018, 20, 104. |
| [5] | (e) Mei, G.-J.; Li, D.; Zhou, G.-X.; Shi, Q.; Cao, Z.; Shi, F. Chem. Commun. 2017, 53, 10030. |
| [5] | (f) Mei, G.-J.; Bian, C.-Y.; Li, G.-H.; Xu, S.-L.; Zheng, W.-Q.; Shi, F. Org. Lett. 2017, 19, 3219. |
| [5] | (g) Sun, M.; Wan, X.; Zhou, S.-J.; Mei, G.-J.; Shi, F. Chem. Commun. 2019, 55, 1283. |
| [5] | (f) Ismail, S. N. F. B. S.; Yang, B.; Zhao, Y. Org. Lett. 2021, 23, 2884. |
| [6] | (a) Brown, R. W.; Zamani, F.; Gardiner, M. G.; Yu, H.-B.; Pyne, S. G.; Hyland, C. J. T. Chem. Sci. 2019, 10, 9051. |
| [6] | (b) Song, B.-C.; Xie, P.-P.; Li, Y.-Z.; Hao, J.-P.; Wang, L.; Chen, X.-Y.; Xu, Z.-L.; Quan, H.-T.; Lou, L.; Xia, Y.-Z.; Houk, K. N.; Yang, W.-B. J. Am. Chem. Soc. 2020, 142, 9982. |
| [7] | (a) Li, T.-R.; Tan, F.; Lu, L.-Q.; Wei, Y.; Wang, Y.-N.; Liu, Y.-Y.; Yang, Q.-Q.; Chen, J.-R.; Shi, D.-Q.; Xiao, W.-J. Nat. Commun. 2014, 5, 5500. |
| [7] | (b) Wei, Y.; Lu, L.-Q.; Li, T.-R.; Feng, B.; Wang, Q.; Xiao, W.-J.; Alper, H. Angew. Chem., Int. Ed. 2016, 55, 2200. |
| [7] | (c) Wang, Q.; Li, T.-R.; Lu, L.-Q.; Li, M.-M.; Zhang, K.; Xiao, W.-J. J. Am. Chem. Soc. 2016, 138, 8360. |
| [7] | (d) Li, M.-M.; Wei, Y.; Liu, J.; Chen, H.-W.; Lu, L.-Q.; Xiao, W.-J. J. Am. Chem. Soc. 2017, 139, 14707. |
| [7] | (e) Feng, B.; Lu, L.-Q.; Chen, J.-R.; Feng, G.-Q.; He, B.-Q.; Lu, B.; Xiao, W.-J. Angew. Chem., Int. Ed. 2018, 57, 5888. |
| [7] | (f) Wei, Y.; Liu, S.; Li, M.-M.; Li, Y.; Lan, Y.; Lu, L.-Q.; Xiao, W.-J. J. Am. Chem. Soc. 2019, 141, 133. |
| [7] | (g) Wang, Y.-N.; Xiong, Q.; Lu, L.-Q.; Zhang, Q.-L.; Wang, Y.; Lan, Y.; Xiao, W.-J. Angew. Chem., Int. Ed. 2019, 58, 11013. |
| [7] | (h) Zhang, M.-M.; Wang, Y.-N.; Wang, B.-C.; Chen, X.-W.; Lu, L.-Q.; Xiao, W.-J. Nat. Commun. 2019, 10, 2716. |
| [7] | (i) Xiong, W.; Xuan, J.; Zhang, M.-M.; Xiao, W.-J.; Lu, L.-Q. Chem. Commun. 2021, 57, 13566. |
| [8] | Zhou, H.-B.; Alper, H. J. Org. Chem. 2003, 68, 3439. |
| [9] | (a) Larksarp, C.; Alper, H. J. Am. Chem. Soc. 1997, 119, 3709. |
| [9] | (b) Larksarp, C.; Alper, H. J. Org. Chem. 1999, 64, 4152. |
| [9] | (c) Trost, B. M.; Fandrick, D. R. J. Am. Chem. Soc. 2003, 125, 11836. |
| [9] | (d) Dong, C.; Alper, H. Tetrahedron: Asymmetry 2004, 15, 1537. |
| [9] | (e) Shintani, R.; Park, S.; Shirozu, F.; Murakami, M.; Hayashi, T. J. Am. Chem. Soc. 2008, 130, 16174. |
| [9] | (f) Shintani, R.; Tsuji, T.; Park, S.; Hayashi, T. J. Am. Chem. Soc. 2010, 132, 7508. |
| [9] | (g) Shintani, R.; Ito, T.; Nagamoto, M.; Otomo, H.; Hayashi, T. Chem. Commun. 2012, 48, 9936. |
| [9] | (h) Khan, A.; Xing, J.-X.; Zhao, J.-M.; Kan, Y.-H.; Zhang, W.-B.; Zhang, Y.-J. Chem.-Eur. J. 2015, 21, 120. |
| [9] | (i) Khan, I.; Shah, B. H.; Zhao, C.; Xu, F.; Zhang, Y.-J. Org. Lett. 2019, 21, 9452. |
| [9] | (j) Hang, Q.-Q.; Liu, S.-J.; Sun, T.-T.; Zhang, Y.-C.; Mei, G.-J.; Shi, F. Chin. J. Chem. 2020, 38, 1612. |
| [10] | Zhang, Q.-L.; Xiong, Q.; Li, M.-M.; Xiong, W.; Shi, B.; Lan, Y.; Lu, L.-Q.; Xiao, W.-J. Angew. Chem., Int. Ed. 2020, 59, 14096. |
/
| 〈 |
|
〉 |