

硫代磺酸酯和磺酰卤的绿色合成研究
收稿日期: 2022-05-07
修回日期: 2022-08-01
网络出版日期: 2022-08-25
基金资助
新疆维吾尔自治区高校科研计划自然科学重点(XJEDU2020I015)
Green Synthesis of Thiosulfonates and Sulfonyl Halides
Received date: 2022-05-07
Revised date: 2022-08-01
Online published: 2022-08-25
Supported by
Scientific Research Program of the Higher Education Institution of Xinjiang(XJEDU2020I015)
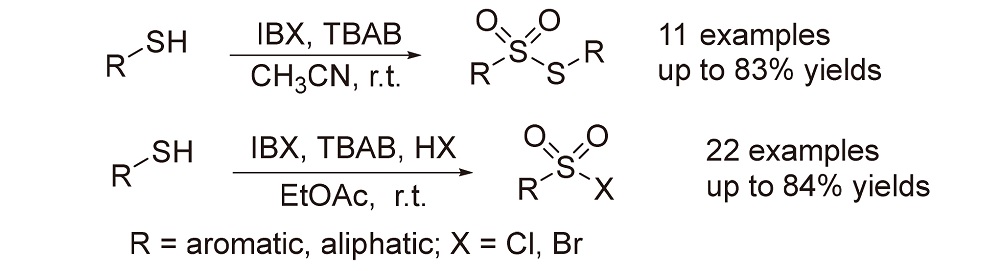
利用2-碘酰基苯甲酸(IBX)的氧化作用, 在四丁基溴化铵(TBAB)作为添加剂的条件下, 硫酚、硫醇类化合物在室温快速反应生成相应的硫代磺酸酯类化合物, 共获得了11个硫代磺酸酯, 产率为54%~83%; 而该氧化体系在加入浓盐酸(w=36%)或浓氢溴酸(w=46%)的条件下, 硫酚或硫醇均顺利被氧化卤代成相应的磺酰氯或磺酰溴, 分别获得了11个磺酰氯和11个磺酰溴产物, 产率为62%~84%. 这两种体系都简单、高效, 使用了绿色氧化剂和溶剂、具有处理简单、条件温和等优点, 所有合成产物均经1H NMR、13C NMR结构确证.
关键词: 一锅法; 2-碘酰基苯甲酸(IBX); 卤化; 绿色合成
乃比江•赛米 , 张蕾 , 买地娜•沙拉木 , 曾竟 , 阿布都热西提•阿布力克木 . 硫代磺酸酯和磺酰卤的绿色合成研究[J]. 有机化学, 2023 , 43(1) : 236 -243 . DOI: 10.6023/cjoc202205010
Using the oxidation between 2-iodoylbenzoic acid (IBX) and tetrabutylammonium bromide (TBAB), thiophenols and thiols reacted rapidly to form corresponding thiosulfonates at room temperature, and 11 thiosulfonates were obtained with yields ranging from 54% to 83%. It was also found that the catalytic system in the presence of aqueous HCl (w=36%) and HBr (w=46%) oxidatively halogenated thiophenols and thiols, respectively, 11 kinds of sulfonyl chlorides and 11 kinds of sulfonyl bromides were obtained with yields ranging from 62% to 84%. The method has the advantages of simple and efficient, simple post-treatment, green oxidant and solvent, mild conditions, etc. All products were confirmed by 1H NMR, 13C NMR structure.

| [1] | Weidner, J. P.; Block, S. S. J. Med. Chem. 1964, 7, 671. |
| [2] | Meng, Y.; Wang, M.; Jiang, X. CCS Chem. 2021, 3, 1735. |
| [3] | Li, Y.; Wang, M.; Jiang, X. Org. Lett. 2021, 23, 4657. |
| [4] | Sohrabnezhad, S.; Bahrami, K.; Hakimpoor, F. J. Sulfur Chem. 2019, 40, 1. |
| [5] | Chen, R.; Xu, S.; Shen, F.; Xu, C.; Wang, K.; Wang, Z.; Liu, L. Molecules 2021, 26, 5551. |
| [6] | Chau, M. M.; Kice, J. L. J. Am. Chem. Soc. 1976, 98, 7711. |
| [7] | Cai, M.-T.; Lv, G.-S.; Chen, J.-X.; Gao, W.-X.; Ding, J.-C.; Wu, H.-Y. Chem. Lett. 2010, 39, 368. |
| [8] | Sobhani, S.; Aryanejad, S.; Maleki, M. Synlett 2011, 319. |
| [9] | Bahrami, K.; Khodaei, M. M.; Khaledian, D. Tetrahedron. Lett. 2012, 53, 354. |
| [10] | Liang, G. G.; Liu, M, C.; Chen, J. X.; Ding, J. C.; Gao, W. X.; Wu, H. Y. Chin. J. Chem. 2012, 30, 1611. |
| [11] | Shyam, P. K.; Kim, Y. K.; Lee, C.; Jang, H.-Y. Adv. Synth. Catal. 2016, 358, 56. |
| [12] | Yang, Z.; Shi, Y.; Zhan, Z.; Zhang, H.; Xing, H.; Lu, R.; Zhang, Y.; Guan, M.; Wu, Y. ChemElectroChem 2018, 5, 3619. |
| [13] | Zhang, X.; Cui, T.; Zhang, Y.; Gu, W.; Liu, P.; Sun, P. Adv. Synth. Catal. 2019, 361, 2014. |
| [14] | Jiang, X. Y.; Yao, C. S.; Tong, C.; Bai, R. R.; Zhou, T.; Xie, Y. Y. Chin. J. Org. Chem. 2020, 40, 1752. (in Chinese) |
| [14] | (蒋筱莹, 姚传胜, 童踔, 白仁仁, 周涛, 谢媛媛, 有机化学, 2020, 40, 1752.) |
| [15] | Bahrami, K.; Khodaei, M. M.; Soheilizad, M. J. Org. Chem. 2009, 74, 9287. |
| [16] | Veisi, H.; Sedrpoushan, A.; Hemmati, S.; Kordestani, D. Phosphorus, Sulfur Silicon Relat. Elem. 2012, 187, 769. |
| [17] | Madabhushi, S.; Jillella, R.; Sriramoju, V.; Singh, R. Green. Chem. 2014, 16, 3125. |
| [18] | Jereb, M.; Hribernik, L. Green Chem. 2017, 19, 2286. |
| [19] | Silva-Cuevas, C.; Perez-Arrieta, C.; Polindara-García, L. A.; Lujan- Montelongo, J. A. Tetrahedron Lett. 2017, 58, 2244. |
| [20] | Liu, S. Z.; Rexit, A. A. Chem. Bull. 2019, 82, 270. (in Chinese) |
| [20] | (刘世钊, 阿布都热西提?阿布力克木, 化学通报, 2019, 82, 270.) |
| [21] | Lü, J.; Zeng, J.; Rexit, A. A. Chin. J. Org. Chem. 2020, 40, 2483. (in Chinese) |
| [21] | (吕进强, 曾竟, 阿布都热西提?阿布力克木, 有机化学, 2020, 40, 2483.) |
/
| 〈 |
|
〉 |