

红树来源真菌Daldinia eschscholtzii HJ004发酵产物中三个新的次级代谢产物
收稿日期: 2022-05-23
修回日期: 2022-07-11
网络出版日期: 2022-08-25
基金资助
海南省重点研发计划(ZDYF2021SHFZ270); 国家自然科学基金(32160108); 国家自然科学基金(41866005); 海南省自然科学基金(220RC593); 海南省科技攻关计划(ZDKJ202008); 海南省院士创新平台海南省专项(YSPTZX202030)
Three New Secondary Metabolites from the Mangrove-Derived Fungus Daldinia eschscholtzii HJ004
Received date: 2022-05-23
Revised date: 2022-07-11
Online published: 2022-08-25
Supported by
Key Research and Development Program of Hainan Province(ZDYF2021SHFZ270); National Natural Science Foundation of China(32160108); National Natural Science Foundation of China(41866005); Natural Science Foundation of Hainan Province(220RC593); Key Science and Technology Program of Hainan Province(ZDKJ202008); Specific Research Fund of the Innovation Platform for Academicians of Hainan Province(YSPTZX202030)
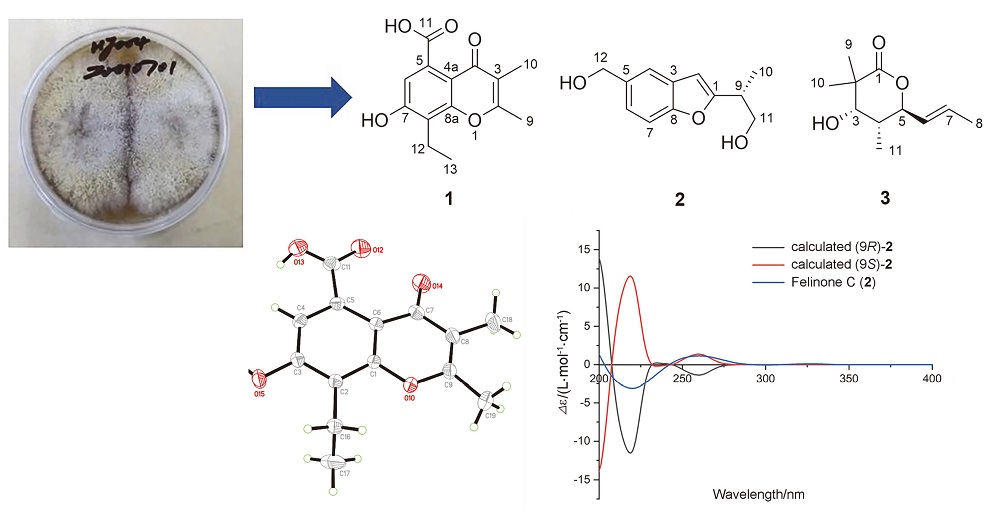
从红树来源真菌Daldinia eschscholtzii HJ004中分离得到3个新化合物以及8个已知化合物. 通过高分辨质谱、一维和二维核磁共振波谱以及X射线单晶衍射数据确定了8-乙基-7-羟基-5-羧基-2,3-二甲基色酮(1)的结构. 通过电子圆二色谱(ECD)量子化学计算以及圆二色谱(CD)数据与文献进行比较, 确定了felinone C (2)和螺旋霉素G (3)的绝对构型. 利用总抗氧化能力检测试剂盒(ABTS快速法)测试了所得11个化合物的自由基清除能力, 其中4个化合物表现出显著的抗氧化活性, IC50值的范围为5.57~195.03 μmol/L, 强于阳性对照水溶性维生素E (trolox)的抗氧化活性(IC50=292.12 μmol/L), 具有开发为抗氧剂的潜力.
关键词: Daldinia eschscholtzii; 色原酮; 内酯类化合物; 抗氧化活性
王斌 , 曾尾女 , 李杲钰 , 肖媚 , 韦方芳 , 罗由萍 , 钮智刚 , 黄国雷 , 郑彩娟 . 红树来源真菌Daldinia eschscholtzii HJ004发酵产物中三个新的次级代谢产物[J]. 有机化学, 2023 , 43(1) : 332 -337 . DOI: 10.6023/cjoc202205041
Three new compounds, together with eight known compounds, were isolated from the mangrove-derived fungus Daldinia eschscholtzii HJ004. The structure of 8-ethyl-7-hydroxy-5-carboxyl-2,3-dimethylchromone (1) was extensively elucidated and characterized by HRESIMS, 1D and 2D NMR, and single-crystal X-ray crystallography. The absolute configurations of felinone C (2) and helicascolide G (3) were determined by using electronic circular dichroism (ECD) method or comparing circular dichroism (CD) data with the literature. The radical scavenging capabilities of the 11 compounds obtained were tested by total antioxidant capacity assay kit with a rapid ABTS method. Among them 4 compounds exhibited stronger antioxidant activity with IC50 values range from 5.57 μmol/L to 195.03 μmol/L, than the positive control trolox (IC50=292.12 μmol/L). These results indicate that chromones have the potential values of developing antioxidants.

Key words: Daldinia eschscholtzii; chromone; lactone; antioxidant activity
| [1] | Chen, S. H.; Cai, R. L.; Liu, Z. M.; Cui, H.; She, Z. G. Nat. Prod. Rep. 2022, 39, 560. |
| [2] | Deshmukh, S. K.; Gupta, M. K.; Prakash, V.; Reddy, M. S. J. Fungi 2018, 4, 101. |
| [3] | Li, S. J.; Jiao, F. W.; Li, W.; Zhang, X.; Yan, W.; Jiao, R. H. J. Nat. Prod. 2020, 83, 2976. |
| [4] | Carroll, A. R.; Copp, B. R.; Davis, R. A.; Keyzers, R. A.; Prinsep, M. R. Nat. Prod. Rep. 2021, 38, 362. |
| [5] | Guo, H. X.; Huang, C. Y.; Yan, Z. Y.; Chen, T.; Hong, K.; Long, Y. H. Chin. J. Nat. Med. 2020, 18, 855. |
| [6] | Zhang, Y. L.; Zhang, J.; Jiang, N.; Lu, Y. H.; Wang, L.; Xu, S. H.; Wang, W.; Zhang, G. F.; Xu, Q.; Ge, H. M.; Ma, J.; Song, Y. C.; Tan, R. X. J. Am. Chem. Soc. 2011, 133, 5931. |
| [7] | Zhang, A. H.; Jiang, N.; Wang, X. Q.; Tan, R. X. Sci. Rep. 2019, 9, 1. |
| [8] | Du, L.; King, J. B.; Cichewicz, R. H. J. Nat. Prod. 2014, 77, 2454. |
| [9] | Lin, L. P.; Tan, R. X. Chin. J. Chem. 2018, 36, 749. |
| [10] | Qin, X. D.; Dong, Z. J.; Liu, J. K.; Yang, L. M.; Wang, R. R.; Zheng, Y. T.; Lu, Y.; Wu, Y. S.; Zheng, Q. T. Helv. Chim. Acta 2006, 89, 127. |
| [11] | Mei, R. Q.; Huang, G. L.; Wang, B.; Bai, M.; Luo, Y. P.; Chen, G. Y.; Zheng, C. J. Chin. J. Org. Chem. 2019, 39, 1479. (in Chinese) |
| [11] | (梅荣清, 黄国雷, 王斌, 白猛, 罗由萍, 陈光英, 郑彩娟, 有机化学, 2019, 39, 1479.) |
| [12] | Bai, M.; Zheng, C. J.; Huang, G. L.; Mei, R. Q.; Wang, B.; Luo, Y. P.; Zheng, C.; Niu, Z. G.; Chen, G. Y. J. Nat. Prod. 2019, 82, 1155. |
| [13] | Liao, H. X.; Shao, T. M.; Mei, R. Q.; Huang, G. L.; Zhou, X. M.; Zheng, C. J.; Wang, C. Y. Mar. Drugs 2019, 17, 710. |
| [14] | Liao, H. X.; Zheng, C. J.; Huang, G. L.; Mei, R. Q.; Nong, X. H.; Shao, T. M.; Chen, G. Y.; Wang, C. Y. J. Nat. Prod. 2019, 82, 2211. |
| [15] | Du, F. Y.; Li, X. M.; Zhang, P.; Li, C. S.; Wang, B. G. Mar. Drugs 2014, 12, 2816. |
| [16] | Poch, G. K.; Gloer, J. B. J. Nat. Prod. 1989, 52, 257. |
| [17] | Rao, C. R.; Venkateswarlu, V. Recl. Trav. Chim. Pays-Bas 1956, 75, 1321. |
| [18] | Dai, J. Q.; Krohn, K.; Floerke, U.; Draeger, S.; Schulz, B.; Kiss-Szikszai, A.; Antus, S.; Kurtan, T.; Van Ree, T. Eur. J. Org. Chem. 2006, 2006, 3498. |
| [19] | Yang, W. C.; Chen, Y.; Cai, R. L.; Zou, G.; Wang, B.; She, Z. G. Chem. Biodiversity 2020, 17, e2000192. |
| [20] | Kato, H.; Li, W.; Koike, M.; Wang, Y. H.; Koike, K. Phytochemistry 2010, 71, 1925. |
| [21] | Cao, F.; Yang, Q.; Shao, C. L.; Kong, C. J.; Zheng, J. J.; Liu, Y. F.; Wang, C. Y. Mar. Drugs 2015, 13, 4171. |
| [22] | Zhang, P.; Meng, L. H.; Attila, M.; Li, X. M.; Tibor, K.; Wang, B. G. RSC Adv. 2015, 5, 39870. |
| [23] | Chen, Y.; Meng, G. L.; Bai, W. L.; Ma, Y.; Xie, L. P.; Altaf, N.; Qian, Y. N.; Han, Y.; Ji, Y. Clin. Exp. Pharmacol. Physiol. 2017, 44, 266. |
/
| 〈 |
|
〉 |