

α-氰醇甲磺酸酯与丙二酸酯的亲核取代反应合成α-芳基腈类化合物
收稿日期: 2022-08-23
修回日期: 2022-10-03
网络出版日期: 2022-11-01
基金资助
国家自然科学基金(21602144); 湖北省教育厅科研计划(Q20211503)
Synthesis of α-Aryl Nitriles via Nucleophilic Substitution of α-Cyanohydrin Methanesulfonates with Malonates
Received date: 2022-08-23
Revised date: 2022-10-03
Online published: 2022-11-01
Supported by
National Natural Science Foundation of China(21602144); Scientific Research Project of Education Department of Hubei Province(Q20211503)
王雷刚 , 郑逸轩 , 周希 , 王海峰 , 严琼姣 , 汪伟 , 陈芬儿 . α-氰醇甲磺酸酯与丙二酸酯的亲核取代反应合成α-芳基腈类化合物[J]. 有机化学, 2023 , 43(2) : 668 -678 . DOI: 10.6023/cjoc202208029
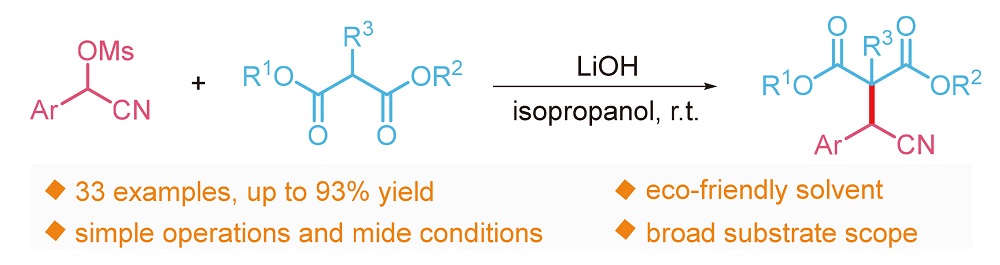
An efficient synthesis of α-aryl nitriles via nucleophilic substitution of α-cyanohydrin methanesulfonates with malonates is developed. This transition metal-free protocol has the advantages of cheap and easily available starting materials, mild reaction conditions, simple operation, a broad substrate scope and high functional group tolerance. Furthermore, this strategy could also be used to asymmetric malonates and acyl esters.
| [1] | (a) Fleming, F. F. Nat. Prod. Rep. 1999, 16, 597. |
| [1] | (b) Fleming, F. F.; Yao, L.; Ravikumar, P. C.; Funk, L.; Shook, B. C. J. Med. Chem. 2010, 53, 7902. |
| [1] | (c) Wang, J.; Liu, H. Chin. J. Org. Chem. 2012, 32, 1643. (in Chinese) |
| [1] | (王江, 柳红, 有机化学, 2012, 32, 1643.) |
| [1] | (d) Cohen, D. T.; Buchwald, S. L. Org. Lett. 2015, 17, 202. |
| [1] | (e) Yan, G.; Zhang, Y.; Wang, J. Adv. Synth. Catal. 2017, 359, 4068. |
| [1] | (f) Que, X.; Qiu, Z.; Yan, Y. High Perform. Polym. 2019, 31, 1062. |
| [2] | (a) Daw, P.; Sinha, A.; Rahaman, S. M. W.; Dinda, S.; Bera, J. K. Organometallics 2012, 31, 3790. |
| [2] | (b) Kumar, S.; Dixit, S. K.; Awasthi, S. K. Tetrahedron Lett. 2014, 55, 3802. |
| [2] | (c) Tomás-Mendivil, E.; Suárez, F. J.; Díez, J.; Cadierno, V. Chem. Commun. 2014, 50, 9661. |
| [2] | (d) Manikandan, R.; Anitha, P.; Prakash, G.; Vijayan, P.; Viswanathamurthi, P.; Butcher, R. J.; Malecki, J. G. J. Mol. Catal. A: Chem. 2015, 398, 312. |
| [2] | (e) Marcé, P.; Lynch, J.; Blacker, A. J.; Williams, J. M. J. Chem. Commun. 2016, 52, 1436. |
| [2] | (f) Singh, K.; Sarbajna, A.; Dutta, I.; Pandey, P.; Bera, J. K. Chem. Eur. J. 2017, 23, 7761. |
| [2] | (g) Ji, P.; Manna, K.; Lin, Z.; Feng, X.; Urban, A.; Song, Y.; Lin, W. J. Am. Chem. Soc. 2017, 139, 7004. |
| [2] | (h) Paul, A.; Chandak, H. S.; Ma, L.; Seidel, D. Org. Lett. 2020, 22, 976. |
| [2] | (i) Li, C.; Chang, X.-Y.; Huo, L.; Tan, H.; Xing, X.; Xu, C. ACS Catal. 2021, 11, 8716. |
| [2] | (j) Tong, S.; Li, K.; Ouyang, X.; Song, R.; Li, J. Green Synth. Catal. 2021, 2, 145. |
| [3] | Jackson, T.; Woo, L. W. L.; Trusselle, M. N.; Chander, S. K.; Purohit, A.; Reed, M. J.; Potter, B. V. L. Org. Biomol. Chem. 2007, 5, 2940. |
| [4] | Teodori, E.; Dei, S.; Garnier-Suillerot, A.; Gualtieri, F.; Manetti, D.; Martelli, C.; Romanelli, M. N.; Scapecchi, S.; Sudwan, P.; Salerno, M. J. Med. Chem. 2005, 48, 7426. |
| [5] | Noble, S.; McTavish, D. Drugs 1995, 50, 1032. |
| [6] | Culkin, D. A.; Hartwig, J. F. J. Am. Chem. Soc. 2002, 124, 9330. |
| [7] | Jiao, Z.; Chee, K. W.; Zhou, J. S. J. Am. Chem. Soc. 2016, 138, 16240. |
| [8] | Qian, X.; Han, J.; Wang, L. Adv. Synth. Catal. 2016, 358, 940. |
| [9] | He, A.; Falck, J. R. J. Am. Chem. Soc. 2010, 132, 2524. |
| [10] | Nambo, M.; Yar, M.; Smith, J. D.; Crudden, C. M. Org. Lett. 2015, 17, 50. |
| [11] | Choi, J.; Fu, G. C. J. Am. Chem. Soc. 2012, 134, 9102. |
| [12] | Kadunce, N. T.; Reisman, S. E. J. Am. Chem. Soc. 2015, 137, 10480. |
| [13] | (a) Wheeler, C.; West, K. N.; Liotta, C. L.; Eckert, C. A. Chem. Commun. 2001, 887. |
| [13] | (b) Kim, D. W.; Song, C. E.; Chi, D. Y. J. Org. Chem. 2003, 68, 4281. |
| [14] | Ratani, T. S.; Bachman, S.; Fu, G. C.; Peters, J. C. J. Am. Chem. Soc. 2015, 137, 13902. |
| [15] | Chen, G.; Wang, Z.; Wu, J.; Ding, K. Org. Lett. 2008, 10, 4573. |
| [16] | Shirsath, S. R.; Shinde, G. H.; Shaikh, A. C.; Muthukrishnan, M. J. Org. Chem. 2018, 83, 12305. |
| [17] | Liu, S.; Meng, L.; Zeng, X.; Hammond, G. B.; Xu, B. Chin. J. Chem. 2021, 39, 913. |
| [18] | (a) Ambro?ak, A.; Steinebach, C.; Gardner, E. R.; Beedie, S. L.; Schnakenburg, G.; Figg, W. D.; Gütschow, M. ChemMedChem 2016, 11, 2621. |
| [18] | (b) Singh, A.; Hakk, H.; Lupton, S. J. J. Labelled Compd. Radiopharm. 2018, 61, 386. |
| [18] | (c) Wang, L.-L.; Battini, N.; Bheemanaboina, R. R. Y.; Zhang, S.-L.; Zhou, C.-H. Eur. J. Med. Chem. 2019, 167, 105. |
| [19] | (a) Kiledal, S. A.; Jourdain, R.; Vellalath, S.; Romo, D. Org. Lett. 2021, 23, 6622. |
| [19] | (b) Cheng, F.; Chen, T.; Huang, Y.-Q.; Li, J.-W.; Zhou, C.; Xiao, X.; Chen, F.-E. Org. Lett. 2022, 24, 115. |
| [19] | (c) Moghadam, F. A.; Hicks, E. F.; Sercel, Z. P.; Cusumano, A. Q.; Bartberger, M. D.; Stoltz, B. M. J. Am. Chem. Soc. 2022, 144, 7983. |
| [19] | (d) Wu, J.; Young, C. M.; Watts, A. A.; Slawin, A. M. Z.; Boyce, G. R.; Bühl, M.; Smith, A. D. Org. Lett. 2022, 24, 4040. |
| [19] | (e) Cochrane, S. R.; Kerr, M. A. Org. Lett. 2022, 24, 5509. |
| [20] | (a) Ueda, M.; Nishimura, K.; Ryu, I. Synlett 2013, 24, 1683. |
| [20] | (b) Wu, G.; Xu, S.; Deng, Y.; Wu, C.; Zhao, X.; Ji, W.; Zhang, Y.; Wang, J. Tetrahedron 2016, 72, 8022. |
| [20] | (c) Li, C.; Zhang, Y.; Sun, Q.; Gu, T.; Peng, H.; Tang, W. J. Am. Chem. Soc. 2016, 138, 10774. |
/
| 〈 |
|
〉 |