

四氢-β-咔啉衍生物的设计、合成及抗氧化性能研究
收稿日期: 2023-01-08
修回日期: 2023-03-19
网络出版日期: 2023-04-14
基金资助
河南农业大学拔尖人才(30501028); 河南省自然科学基金(232300420376)
Design, Synthesis and Antioxidant Activity of Tetrahydro-β-carbolines
Received date: 2023-01-08
Revised date: 2023-03-19
Online published: 2023-04-14
Supported by
The Top-Notch Personnel Program of Henan Agricultural University(30501028); The Natural Science Foundation of Henan Province(232300420376)
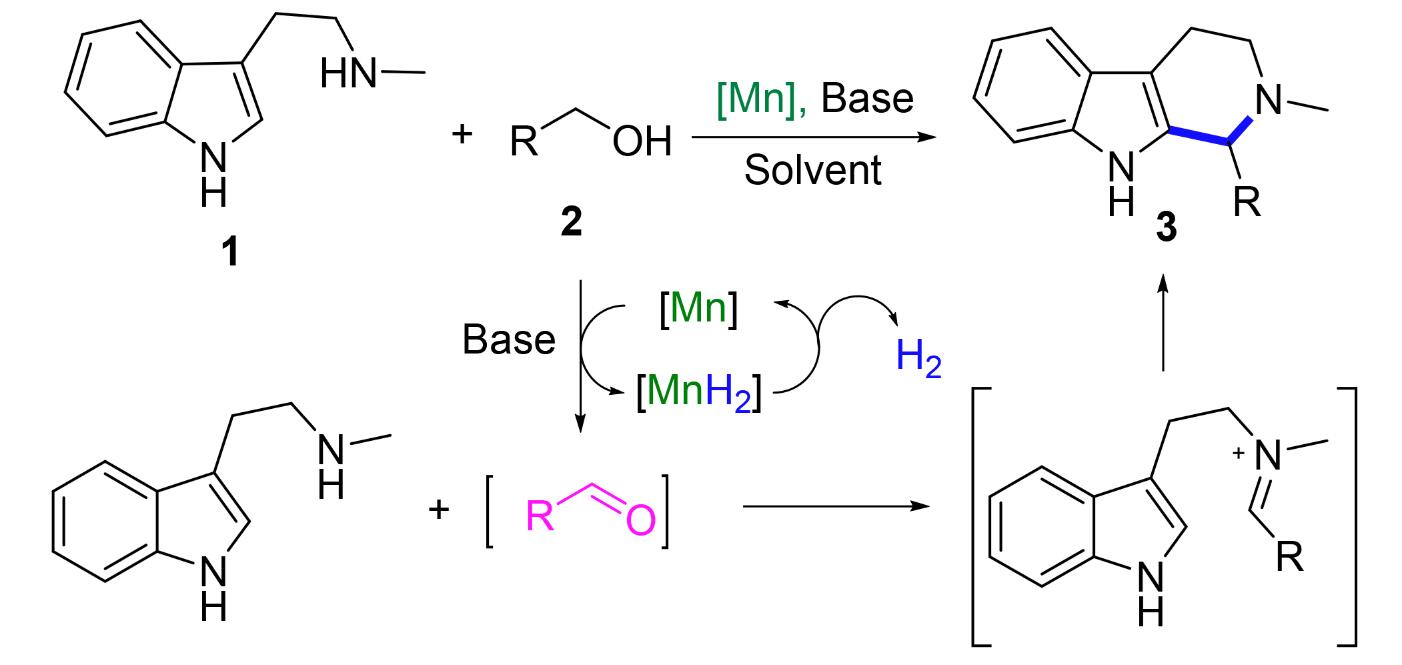
四氢-β-咔啉类化合物(THBCs)是吲哚类生物碱的重要结构单元, 大多具有显著的抗炎、抗病毒、抗癌、抗菌及抗氧化等生物特性. 以3-(2-甲基氨基乙基)吲哚和醇为起始原料, 发展了一种钳形锰催化剂催化的氧化Pictet-Spengler反应. 该反应体系具有良好的底物适用性, 以芳香醇和脂肪醇为底物合成了12种THBCs产物, 收率为43%~99%. 并采用1H NMR、13C NMR和HRMS等方法对产物的结构进行确认和表征. 同时通过清除ABTS+•和DPPH•实验研究了此类化合物的抗氧化能力. 结果表明, 在2个测试体系中, 12种化合物均具有一定的自由基清除能力, 说明这些化合物是一类潜在的抗氧化剂. 其中, 1-(4-氟苯基)-2-甲基-2,3,4,9-四氢-1H-吡啶-[3,4-b]吲哚(3c)、2-甲基-1-(2-萘基)-2,3,4,9-四氢-1H-吡啶-[3,4-b]吲哚(3e)、1-丁基-2-甲基-2,3,4,9-四氢-1H-吡啶-[3,4-b]吲哚(3h)、(E)-2-甲基-1-(3-戊烯基)-2,3,4,9-四 氢-1H-吡啶-[3,4-b]吲哚(3j)、2-甲基-1-(2-吡啶-3-乙基)-2,3,4,9-四氢-1H-吡啶-[3,4-b]吲哚(3l)清除ABTS+•效果最佳, 1-环己基-2-甲基-2,3,4,9-四氢-1H-吡啶-[3,4-b]吲哚(3i)、2-甲基-1-(2-噻吩-2-乙基)-2,3,4,9-四氢-1H-吡啶-[3,4-b]吲哚(3k)稍弱, 2-甲基-1-苯基-2,3,4,9-四氢-1H-吡啶-[3,4-b]吲哚(3a)、2-甲基-1-(4-甲硫苯基)-2,3,4,9-四氢-1H-吡啶- [3,4-b]吲哚(3b)、1-(2-呋喃基)-2-甲基-2,3,4,9-四氢-1H-吡啶-[3,4-b]吲哚(3d)、2-甲基-1-(3-吡啶基)-2,3,4,9-四氢-1H-吡啶-[3,4-b]吲哚(3f)、2-甲基-1-(2-噻吩基)-2,3,4,9-四氢-1H-吡啶-[3,4-b]吲哚(3g)次之, IC50可达0.073 mg•mL-1及以上. 目标化合物清除DPPH•的能力以化合物3a、3c、3e、3h的效果最佳, 3d、3i、3j、3l次之, 3b、3f、3g效果最差. 综上, 化合物3c、3e、3h对ABTS+•和DPPH•均有较好的清除能力, 3b、3f、3g对2,2'-偶氮-双(3-乙基苯并噻唑啉-6-磺酸)二铵盐自由基(ABTS+•)以及2,2'-偶氮-双(3-乙基苯并噻唑啉-6-磺酸)二铵盐自由基(DPPH•)的清除能力均较差.
关键词: 四氢-β-咔啉; 锰催化剂; 醇; Pictet-Spengler反应; 抗氧化
张晓雨 , 李欣燕 , 崔冰 , 邵志晖 , 赵铭钦 . 四氢-β-咔啉衍生物的设计、合成及抗氧化性能研究[J]. 有机化学, 2023 , 43(8) : 2885 -2894 . DOI: 10.6023/cjoc202301007
Tetrahydro-β-carbolines (THBCs) are important structural units of indole alkaloids, most of which have significant biological properties, such as anti-inflammatory, antiviral, anticancer, antibacterial, and antioxidant properties. Herein, a Pictet-Spengler oxidation reaction with 3-(2-methylaminoethyl) indole and alcohols as starting materials was developed over the pincer manganese catalyst. The reaction system showed good substrate applicability, 12 kinds of THBC products were synthesized from aromatic alcohols and aliphatic alcohols with the yields of 43%~99%. The structures of the products were confirmed and characterized by 1H NMR, 13C NMR, IR and HRMS. The antioxidant activity of these compounds were investigated by scavenging ABTS+• and DPPH•. The results showed that in the two test systems, all of those compounds had good free radical scavenging ability, indicating that those compounds are a class of potential antioxidant. Among them, 1-(4-fluoro- phenyl)-2-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (3c), 2-methyl-1-(naphthalen-2-yl)-2,3,4,9-tetrahydro-1H-pyrido- [3,4-b]indole (3e), 2-methyl-1-propyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole(3h), 2-methyl-1-propyl-2,3,4,9-tetrahydro- 1H-pyrido[3,4-b]indole (3j) and 2-methyl-1-(2-(pyridin-3-yl)ethyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (3l) showed the best effect on scavenging ABTS+•, while 1-cyclohexyl-2-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (3i) and 2-methyl- 1-(2-(thiophen-2-yl)ethyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (3k) were slightly weaker, followed by 2-methyl-1-phe- nyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (3a), 2-methyl-1-(4-(methylthio)phenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]- indole (3b), 1-(furan-2-yl)-2-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (3d), 2-methyl-1-(pyridin-3-yl)-2,3,4,9- tetrahydro-1H-pyrido[3,4-b]indole (3f) and 2-methyl-1-(thiophen-2-yl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole (3g), and IC50 could reach 0.073 mg•mL-1 or above. Among of the target compounds, 3a, 3c, 3e and 3h had the best effect on scavenging DPPH•, followed by 3d, 3i, 3j and 3l, and 3b, 3f and 3g had the worst effect. In conclusion, compounds 3c, 3e and 3h had good scavenging ability on ABTS+• and DPPH•, while compounds 3b, 3f and 3g had poor scavenging ability on 2,2'-azino- bis(3-ethylbenzothiazoline-6-sulfonate) cationic radical (ABTS+•) and 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH•).

| [1] | Daugan, A.; Grondin, P.; Ruault, C.; Le Monnier de Gouville, A.-C.; Coste, H.; Kirilovsky, J.; Hyafil, F.; Labaudinière, R. J. Med. Chem. 2003, 46, 4525. |
| [2] | Miller, J. F.; Turner, E. M.; Sherrill, R. G.; Gudmundsson, K.; Spaltenstein, A.; Sethna, P.; Brown, K. W.; Harvey, R.; Romines, K. R.; Golden, P. Bioorg. Med. Chem. Lett. 2010, 20, 256. |
| [3] | Shankaraiah, N.; Nekkanti, S.; Chudasama, K. J.; Senwar, K. R.; Sharma, P.; Jeengar, M. K.; Naidu, V. G. M.; Srinivasulu, V.; Srinivasulu, G.; Kamal, A. Bioorg. Med. Chem. Lett. 2014, 24, 5413 |
| [4] | Ma, Y.; Wu, H.; Zhang, J.; Li, Y. Chirality 2013, 25, 656. |
| [5] | Goh, T. B.; Koh, R. Y.; Yam, M. F.; Azhar, M. E.; Mordi, M. N.; Mansor, S. M. Food Chem. 2015, 183, 208. |
| [6] | Lim, K.-H.; Komiyama, K.; Kam, T.-S. Tetrahedron Lett. 2007, 48, 1143. |
| [7] | Yamada, H.; Kawate, T.; Matsumizu, M.; Nishida, A.; Yamaguchi, K.; Nakagawa, M. J. Org. Chem. 1998, 63, 6348 |
| [8] | Bou-Hamdan, F. R.; Leighton, J. L. Angew. Chem., Int. Ed. 2009, 48, 2403. |
| [9] | Ryabukhin, S. V.; Panov, D. M.; Plaskon, A. S.; Tolmachev, A. A.; Smaliy, R. V. Monatsh. Chem. 2012, 143, 1507. |
| [10] | Muratore, M. E.; Holloway, C. A.; Pilling, A. W.; Storer, R. I.; Trevitt, G.; Dixon, D. J. J. Am. Chem. Soc. 2009, 131, 10796. |
| [11] | Wendlandt, A. E.; Stahl, S. S. J. Am. Chem. Soc. 2014, 136, 506. |
| [12] | Wanner, M. J.; van der Haas, R. N. S.; de Cuba, K. R.; van Maarseveen, J. H.; Hiemstra, H. Angew. Chem., Int. Ed. 2007, 46, 7485 |
| [13] | Hamid, M. H. S. A.; Slatford, P. A.; Williams, J. M. J. Adv. Synth. Catal. 2007, 349, 1555. |
| [14] | Hollmann, D. ChemSusChem 2014, 7, 2411. |
| [15] | Corma, A.; Navas, J.; Sabater, M. J. Chem. Rev. 2018, 118, 1410. |
| [16] | Nalikezhathu, A.; Cherepakhin, V.; Williams, T. J. Org. Lett. 2020, 22, 4979. |
| [17] | Yang, P.; Zhang, C.; Gao, W.-C.; Ma, Y.; Wang, X.; Zhang, L.; Yue, J.; Tang, B. ChemComm 2019, 55, 7844. |
| [18] | Mukherjee, A.; Nerush, A.; Leitus, G.; Shimon, L. J. W.; Ben David, Y.; Espinosa Jalapa, N. A.; Milstein, D. J. Am. Chem. Soc. 2016, 138, 4298. |
| [19] | Elangovan, S.; Neumann, J.; Sortais, J.-B.; Junge, K.; Darcel, C.; Beller, M. Nat. Commun. 2016, 7, 12641. |
| [20] | Maji, B.; Barman, M. K. Synthesis 2017, 49, 3377. |
| [21] | Bauer, J. O.; Chakraborty, S.; Milstein, D. ACS Catal. 2017, 7, 4462. |
| [22] | Wang, Y.; Shao, Z.; Zhang, K.; Liu, Q. Angew. Chem., Int. Ed. 2018, 57, 15143. |
| [23] | Moulton, C. J.; Shaw, B. L. J. Chem. Soc., Dalton Trans. 1976, 1020. |
| [24] | Sandoval, C. A.; Ohkuma, T.; Mu?iz, K.; Noyori, R. J. Am. Chem. Soc. 2003, 125, 13490. |
| [25] | Ikariya, T.; Murata, K.; Noyori, R. Org. Biomol. Chem. 2006, 4, 393. |
| [26] | Gunanathan, C.; Milstein, D. Chem. Rev. 2014, 114, 12024. |
| [27] | Shao, Z.; Li, Y.; Liu, C.; Ai, W.; Luo, S.-P.; Liu, Q. Nat. Commun. 2020, 11, 591. |
| [28] | Shao, Z.; Wang, Y.; Liu, Y.; Wang, Q.; Fu, X.; Liu, Q. Org. Chem. Front. 2018, 5, 1248. |
| [29] | Liu, Y.; Shao, Z.; Wang, Y.; Xu, L.; Yu, Z.; Liu, Q. ChemSusChem 2019, 12, 3069. |
| [30] | Fu, S.; Shao, Z.; Wang, Y.; Liu, Q. J. Am. Chem. Soc. 2017, 139, 11941. |
| [31] | Shan, H.-Y.; Yu, Y.-J.; Lv, Y.-P. Food Sci. Technol. 2018, 43, 197. (in Chinese) |
| [31] | ( 单虹宇, 于雅静, 吕远平, 食品科技, 2018, 43, 197.) |
| [32] | Zhan, P.-Q.; Zeng, H.-P. Chin. J. Org. Chem. 2008, 1035. (in Chinese) |
| [32] | ( 张培全, 曾和平, 有机化学, 2008, 1035.) |
| [33] | Elderwish, S.; Audebrand, A.; Nebigil, C. G.; Désaubry, L. Eur. J. Med. Chem. 2020, 186, 111859. |
| [34] | Wu, T. Y. H.; Schultz, P. G. Org. Lett. 2002, 4, 4033. |
| [35] | Chan, Y.-C.; Sak, M. H.; Frank, S. A.; Miller, S. J. Angew. Chem., Int. Ed. 2021, 60, 24573. |
| [36] | Cai, J.-H.; Yang, Y.-Q.; Zeng, Y.-F. Mod. Chem. Ind. 2022, 42, 201. (in Chinese) |
| [36] | ( 蔡杰慧, 杨英全, 郑燕菲, 现代化工, 2022, 42, 201.) |
| [37] | Huang, L.-L.; Zheng, Y.; Wang, X.; Feng, L.; Ye, L.; Hu, P.; Yan, X.; Feng, W.-W.; Wang, J.-R.; Xia, H.-L. J. Chin. Pharm. Sci. 2022, 39, 2489. (in Chinese) |
| [37] | ( 黄李璐, 郑雨, 王希, 冯丽, 叶磊, 胡攀, 严鑫, 冯五文, 汪俊汝, 夏厚林, 中国现代应用药学, 2022, 39, 2489.) |
/
| 〈 |
|
〉 |