

钯催化立体选择性合成硝基烷类β-碳糖苷
收稿日期: 2023-03-14
修回日期: 2023-05-08
网络出版日期: 2023-05-30
基金资助
国家自然科学基金(22207063); 高等学校学科创新引智计划(111计划); 高等学校学科创新引智计划(D20015); 湖北省自然科学基金(2022CFB838); 湖北省教育厅(D20221204); 湖北省教育厅(Q20221212)
Pd-Catalyzed Stereoselective Synthesis of Nitroalkyl β-C-Glycosides
Received date: 2023-03-14
Revised date: 2023-05-08
Online published: 2023-05-30
Supported by
National Natural Science Foundation of China(22207063); Programme of Introducing Talents of Discipline to Universities (111 Project); Programme of Introducing Talents of Discipline to Universities(D20015); Hubei Provincial Natural Science Foundation(2022CFB838); Educational Commission of Hubei Province(D20221204); Educational Commission of Hubei Province(Q20221212)
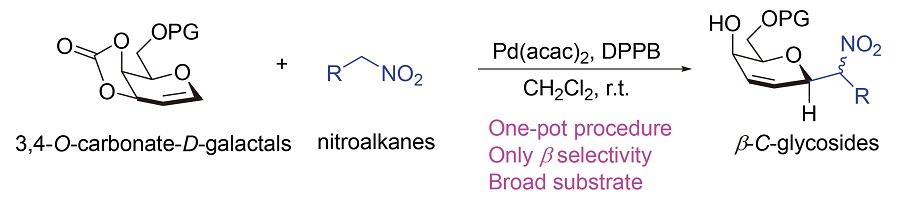
碳糖苷因其优异的生理活性及耐水解/酶解特性, 在医学和生物学领域的研究中受到越来越多的关注, 但合成过程中仍面临着立体选择性的控制等挑战. 报道了一种3,4-O-碳酸酯烯糖和硝基化合物在双乙酰丙酮钯和1,4-双(二苯膦)丁烷(DPPB)配体的催化作用下, 室温反应得到具有高立体选择性的β-碳糖苷的方法, 且已由核磁共振(NMR)、高分辨质谱(HRMS)以及X射线单晶衍射等方法确定目标化合物的结构. 该方法具有广泛的底物范围, 对含吸电子基或供电子基的硝基烷类化合物都有很好的兼容性, 能以高产率得到单一β构型的碳糖苷, 为快速构建碳苷化合物库提供了可靠方法.
王兢睿 , 冯永奎 , 王能中 , 黄年玉 , 姚辉 . 钯催化立体选择性合成硝基烷类β-碳糖苷[J]. 有机化学, 2023 , 43(9) : 3216 -3225 . DOI: 10.6023/cjoc202303019
C-Glycosides have attracted more and more attention in the field of medicine and biology due to their excellent physiological activities and stability to hydrolysis/enzymolysis. However, the challenges of stereoselective control are still high during the synthesis process. In this paper, a method for preparing β-C-glycosides with high stereoselectivity through the reaction of 3,4-O-carbonate-D-galactal and nitroalkane with Pd(acac)2 and 1,4-bis(diphenylphosphino)butane (DPPB) ligand at room temperature has been reported. The structures of the target compounds have been determined by nuclear magnetic resonance spectroscopy (NMR), high-resolution mass spectra (HRMS) and X-ray single crystal diffraction. The method has a wide range of substrates, and has good compatibility for both electron-withdrawing and electron-donating nitroalkanes. Single β-C-glycosides were obtained with high yields, which provide a reliable method for the rapid construction of C-glycoside libraries.

Key words: glycals; stereoselectivity; C-galactosides; C-glycosylation; palladium catalysis
| [1] | (a) Imperiali B. J. Am. Chem. Soc. 2012, 134, 17835. |
| [1] | (b) Ma Z.; Zhang H.; Wang P. G.; Liu X.; Chen M. Oncotarget 2018, 9, 75. |
| [2] | Guo Y.; Qiu Y.; Sun Q.; Luo X. G. Chem. Bull. 2022, 85, 274. (in Chinese) |
| [2] | (郭芸, 邱媛, 孙琦, 罗晓刚, 化学通报, 2022, 85, 274.) |
| [3] | Hu J.; Wu W.; Qin Y.; Liu C.; Wei P.; Hu J.; Seeberger P. H.; Yin J. Adv. Funct. Mater. 2020, 30, 1910084. |
| [4] | Yang Y.; Yu B. Chem. Rev. 2017, 117, 12281. |
| [5] | Yang G.; Schmieg J.; Tsuji M.; Franck R. W. Angew. Chem., Int. Ed. 2004, 43, 3818. |
| [6] | Wipf P.; Pierce J. G. Org. Lett. 2006, 8, 3375. |
| [7] | Aguillon A. R.; Mascarello A.; Segretti N. D.; de Azevedo H. F. Z.; Guimaraes C. R.; Miranda L. S.; de Souza R. O. Org. Process Res. Dev. 2018, 22, 467. |
| [8] | Madaan T.; Akhtar M.; Najmi A. K. Eur. J. Pharm. Sci. 2016, 93, 244. |
| [9] | Wu Z.; Wei G.; Lian G.; Yu B. J. Org. Chem. 2010, 75, 5725. |
| [10] | (a) Kumar L.; Jain A.; Lal N.; Sarswat A.; Jangir S.; Kumar L.; Singh V.; Shah P.; Jain S. K.; Maikhuri J. P.; Siddiqi M. I.; Gupta G.; Sharma V. L. ACS Med. Chem. Lett. 2012, 3, 83. |
| [10] | (b) Li Y.; Liu Y.; Yang Y.; Yu F.; Liu J.; Song H.; Liu J.; Tang H.; Ye B.-C.; Sun Z. ACS Appl. Mater. Interfaces 2015, 7, 15474. |
| [10] | (c) Müller J.; Schildknecht P.; Müller N. J. Antimicrob. Chemother. 2013, 68, 1781. |
| [10] | (d) Freeman C. D.; Klutman N. E.; Lamp K. C. Drugs 1997, 54, 679. |
| [11] | Raj M.; Mahesh S.; Adebomi V.; Muneeswaran Z. P. Angew. Chem., Int. Ed. 2020, 132, 2815. |
| [12] | Romney D. K.; Sarai N. S.; Arnold F. H. ACS Catal. 2019, 9, 8726. |
| [13] | (a) Isobe M.; Phoosaha W.; Saeeng R.; Kira K.; Yenjai C. Org. Lett. 2003, 5, 4883. |
| [13] | (b) Ferrier R. J.; Overend W. G.; Ryan A. E. J. Chem. Soc. 1962, 3667. |
| [13] | (c) Addanki R. B.; Halder S.; Kancharla P. K. Org. Lett. 2022, 24, 1465. |
| [14] | Zeng J.; Vedachalam S.; Xiang S.; Liu X.-W. Org. Lett. 2011, 13, 42. |
| [15] | Liao J.; Liu H.; Sun J. Chin. J. Org. Chem. 2017, 37, 1382. (in Chinese) |
| [15] | (廖进喜, 刘慧, 孙建松, 有机化学, 2017, 37, 1382.) |
| [16] | Xiong D.-C.; Zhang L.-H.; Ye X.-S. Org. Lett. 2009, 11, 1709. |
| [17] | (a) Lai M. N.; Othman K. A.; Yao H.; Wang Q.; Feng Y.; Huang N.; Liu M.; Zou K. Org. Lett. 2020, 22, 1144. |
| [17] | (b) Lai M. N.; Wang Q. Y.; Hua M.; Huang N.; Yao H. Chin. J. Org. Chem. 2022, 42, 1694. (in Chinese) |
| [17] | (来梦楠, 王秋圆, 华敏, 黄年玉, 姚辉, 有机化学, 2022, 42, 1694.) |
| [18] | (a) Wang Q.; Lai M.; Luo H.; Ren K.; Wang J.; Huang N.; Deng Z.; Zou K.; Yao H. Org. Lett. 2022, 24, 1587. |
| [18] | (b) Liu Y.; Jiao Y.; Luo H.; Huang N.; Lai M.; Zou K.; Yao H. ACS Catal. 2021, 11, 5287. |
| [18] | (c) Hou M.; Xiang Y.; Gao J.; Zhang J.; Wang N.; Shi H.; Huang N.; Yao H. Org. Lett. 2023, 25, 832. |
| [19] | (a) Ding W. Y.; Liu H.-H.; Cheng J.; Yao H.; Xiang S.; Tan B. Org. Chem. Front. 2022, 9, 6149. |
| [19] | (b) Ding W. Y.; Zhao H.-W.; Cheng J.; Lu Z.; Xiang S.; Tan B. Org. Lett. 2022, 24, 7031. |
| [19] | (c) Yao H.; Zhang S.; Leng W.-L.; Leow M.-L.; Xiang S.; He J.; Liao H.; Hoang K. L. M.; Liu X.-W. ACS Catal. 2017, 7, 5456. |
/
| 〈 |
|
〉 |